Page 179 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 179
P1: GKY/MBQ P2: GLQ Final Pages
Encyclopedia of Physical Science and Technology en012i-947 July 26, 2001 11:11
Polymers, Inorganic and Organometallic 687
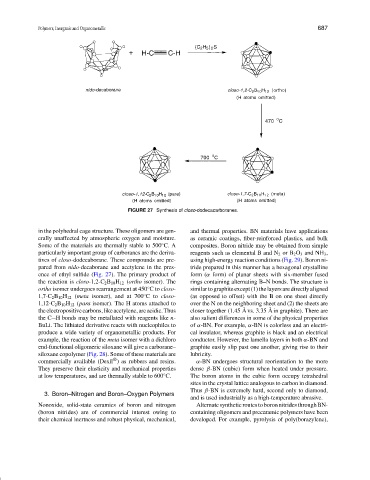
FIGURE 27 Synthesis of closo-dodecacarboranes.
in the polyhedral cage structure. These oligomers are gen- and thermal properties. BN materials have applications
erally unaffected by atmospheric oxygen and moisture. as ceramic coatings, fiber-reinforced plastics, and bulk
◦
Some of the materials are thermally stable to 500 C. A composites. Boron nitride may be obtained from simple
particularly important group of carboranes are the deriva- reagents such as elemental B and N 2 or B 2 O 3 and NH 3 ,
tives of closo-dodecaborane. These compounds are pre- using high-energy reaction conditions (Fig. 29). Boron ni-
pared from nido-decaborane and acetylene in the pres- tride prepared in this manner has a hexagonal crystalline
ence of ethyl sulfide (Fig. 27). The primary product of form (α form) of planar sheets with six-member fused
the reaction is closo-1,2-C 2 B 10 H 12 (ortho isomer). The rings containing alternating B–N bonds. The structure is
◦
ortho isomer undergoes rearrangement at 450 Cto closo- similar to graphite except (1) the layers are directly aligned
◦
1,7-C 2 B 10 H 12 (meta isomer), and at 700 Cto closo- (as opposed to offset) with the B on one sheet directly
1,12-C 2 B 10 H 12 (para isomer). The H atoms attached to over the N on the neighboring sheet and (2) the sheets are
˚
˚
theelectropositivecarbons,likeacetylene,areacidic.Thus closer together (1.45 A vs. 3.35 A in graphite). There are
the C H bonds may be metallated with reagents like n- also salient differences in some of the physical properties
BuLi. The lithiated derivative reacts with nucleophiles to of α-BN. For example, α-BN is colorless and an electri-
produce a wide variety of organometallic products. For cal insulator, whereas graphite is black and an electrical
example, the reaction of the meta isomer with a dichloro conductor. However, the lamella layers in both α-BN and
end-functional oligomeric siloxane will give a carborane– graphite easily slip past one another, giving rise to their
siloxane copolymer (Fig. 28). Some of these materials are lubricity.
®
commercially available (Dexil ) as rubbers and resins. α-BN undergoes structural reorientation to the more
They preserve their elasticity and mechanical properties dense β-BN (cubic) form when heated under pressure.
◦
at low temperatures, and are thermally stable to 600 C. The boron atoms in the cubic form occupy tetrahedral
sites in the crystal lattice analogous to carbon in diamond.
Thus β-BN is extremely hard, second only to diamond,
3. Boron–Nitrogen and Boron–Oxygen Polymers
and is used industrially as a high-temperature abrasive.
Nonoxide, solid-state ceramics of boron and nitrogen Alternate synthetic routes to boron nitrides through BN-
(boron nitrides) are of commercial interest owing to containing oligomers and preceramic polymers have been
their chemical inertness and robust physical, mechanical, developed. For example, pyrolysis of poly(borazylene),