Page 182 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 182
P1: GKY/MBQ P2: GLQ Final Pages
Encyclopedia of Physical Science and Technology en012i-947 July 26, 2001 11:11
690 Polymers, Inorganic and Organometallic
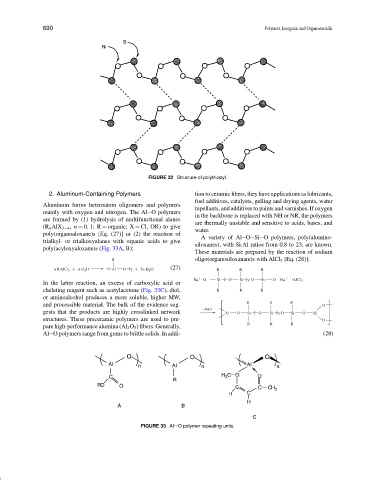
FIGURE 32 Structure of polythiazyl.
2. Aluminum-Containing Polymers tion to ceramic fibers, they have applications as lubricants,
fuel additives, catalysts, gelling and drying agents, water
Aluminum forms heteroatom oligomers and polymers
repellants, and additives to paints and varnishes. If oxygen
mainly with oxygen and nitrogen. The Al O polymers
in the backbone is replaced with NH or NR, the polymers
are formed by (1) hydrolysis of multifunctional alanes
are thermally unstable and sensitive to acids, bases, and
(R n AlX 3−n , n = 0, 1; R = organic; X = Cl, OR) to give
water.
poly(organoaloxane)s [Eq. (27)] or (2) the reaction of
A variety of Al O Si O polymers, poly(alumino-
trialkyl- or trialkoxyalanes with organic acids to give
siloxanes), with Si:Al ratios from 0.8 to 23, are known.
poly(acyloxyaloxane)s (Fig. 33A, B):
These materials are prepared by the reaction of sodium
R oligo(organosiloxanate)s with AlCl 3 [Eq. (28)]:
n RAlCl 3 n H 2 O ( Al O ) n 2n H 2 O (27) R R R
Na O Si ( O Si ) n O Si O Na AICI 3
In the latter reaction, an excess of carboxylic acid or
chelating reagent such as acetylacetone (Fig. 33C), diol, R R R
or aminoalcohol produces a more soluble, higher MW,
and processible material. The bulk of the evidence sug- R R R O
NaCI
gests that the products are highly crosslinked network Al O Si ( O Si ) n O Si O Al
structures. These preceramic polymers are used to pre- O
R R R
pare high-performance alumina (Al 2 O 3 ) fibers. Generally,
Al O polymers range from gums to brittle solids. In addi- (28)
FIGURE 33 Al O polymer repeating units.