Page 280 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 280
P1: GPQ Final Pages/GNB P2: GTV
Encyclopedia of Physical Science and Technology En012c-604 July 26, 2001 16:2
Polymers, Thermally Stable 789
The success of wholly aromatic poly(1,3,4-oxadia- atively low cross-link density (increased toughness), low
zoles) as fiber-forming materials generated interest in moisture absorbtion, and a low dielectric constant. Signif-
the inclusion of the 1,3,4-oxadiazole moiety into ordered icantly it is the relatively high T g values of CE polymers
heterocycle–amide copolymers (XVIII) and wholly or- linked to their hydrophobicity that has been instrumental
dered heterocycle copolymers (XIX). Although “tailored- in generating the major interest in potentially advanced
in” improvements to mechanical properties, tractability, applications.
and even thermal/thermo-oxidative stability have been ob-
served, a relatively poor light stability for these materials N C O R O C N pregel stage
via
has limited useful development.
(XXI)
Analogous homo- and copoly(1,3,4-thiadiazoles, S re-
placing O in the heterocycle ring, are similarly film-
O O
and fiber-forming materials exhibiting somewhat higher C N N C
thermooxidative stability than the 1,3,4-oxadiazoles. A N C O R O C N (XXII)
hydrogen-free alternating imide–thiadiazole copolymer CN NC
O O
(XX) film showed outstanding thermal/thermooxidative
n
stability (weight loss in air and nitrogen and retention of
cross-linked
◦
strength) up to 600 C. CE resin
The low viscosity of monomers and prepolymers facili-
tates processing of CE resins using a variety of traditional
techniques. This ease of processing, allied with their envi-
ronmental stability has, during the 1990s, led to a number
of “high-tech” applications for CE resins both as unre-
2. Aryl Cyanate Esters inforced and as carbon fiber composite materials. They
have proved to be strong candidates in the choice of mate-
The first successful route to the aryl cyanate esters (CEs), rials for a variety of general microelectronics applications
compounds containing the O C N moiety, originated in as well as materials of choice in aerospace applications
the late 1960s via reaction of phenols with cyanogen chlo- including their selection as structural composites in pri-
ride using a technology pioneered by Bayer AG. Further mary and secondary structures in both military and civilian
research and development of this process by Mitsubishi, aircraft.
Gas Chemical Corporation, Rhone Poulenc, Ciba Geigy, Typical resin systems that have demonstrated limited
and Dow Chemical, among others, has provided a vari- commercial success are shown in Table VI, the limita-
ety of commercially available, but relatively high priced, tion being the relatively high price of the resins. Despite
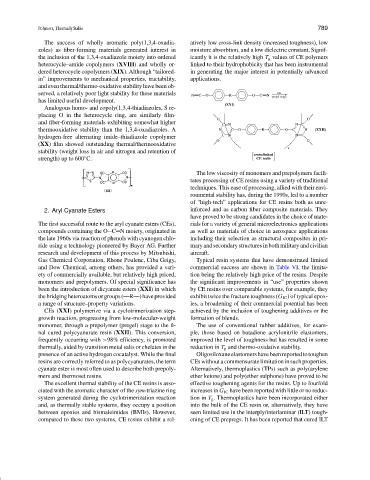
monomers and prepolymers. Of special significance has the significant improvements in “use” properties shown
been the introduction of dicyanate esters (XXI) in which by CE resins over comparable systems, for example, they
thebridgingheteroatomsorgroups(—R—)haveprovided exhibit twice the fracture toughness (G IC ) of typical epox-
a range of structure–property variations. ies, a broadening of their commercial potential has been
CEs (XXI) polymerize via a cyclotrimerization step- achieved by the inclusion of toughening additives or the
growth reaction, progressing from low-molecular-weight formation of blends.
monomer, through a prepolymer (pregel) stage to the fi- The use of conventional rubber additives, for exam-
nal cured polycyanurate resin (XXII). This conversion, ple, those based on butadiene acrylonitrile elastomers,
frequently occurring with >98% efficiency, is promoted improved the level of toughness but has resulted in some
thermally, aided by transition metal salts or chelates in the reduction in T g and thermo-oxidative stability.
presence of an active hydrogen cocatalyst. While the final Oligosiloxaneelastomershavebeenreportedtotoughen
resins are correctly referred to as polycyanurates, the term CEs without a commensurate limitation in such properties.
cyanate ester is most often used to describe both prepoly- Alternatively, thermoplastics (TPs) such as poly(arylene
mers and thermoset resins. ether ketone) and poly(ether sulphone) have proved to be
The excellent thermal stability of the CE resins is asso- effective toughening agents for the resins. Up to fourfold
ciated with the aromatic character of the sym-triazine ring increases in G IC have been reported with little or no reduc-
system generated during the cyclotrimerization reaction tion in T g . Thermoplastics have been incorporated either
and, as thermally stable systems, they occupy a position into the bulk of the CE resin or, alternatively, they have
between epoxies and bismaleimides (BMIs). However, seen limited use in the interply/interlaminar (ILT) tough-
compared to these two systems, CE resins exhibit a rel- ening of CE prepregs. It has been reported that cured ILT