Page 64 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 64
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 879
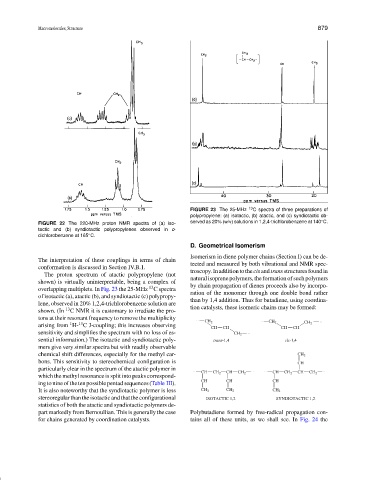
FIGURE 23 The 25-MHz 13 C spectra of three preparations of
polypropylene: (a) isotactic, (b) atactic, and (c) syndiotactic ob-
◦
FIGURE 22 The 220-MHz proton NMR spectra of (a) iso- served as 20% (w/v) solutions in 1,2,4-trichlorobenzene at 140 C.
tactic and (b) syndiotactic polypropylenes observed in o-
dichlorobenzene at 165 C.
◦
D. Geometrical Isomerism
Isomerism in diene polymer chains (Section I) can be de-
The interpretation of these couplings in terms of chain
tected and measured by both vibrational and NMR spec-
conformation is discussed in Section IV.B.1.
troscopy.Inadditiontothecisandtransstructuresfoundin
The proton spectrum of atactic polypropylene (not
natural isoprene polymers, the formation of such polymers
shown) is virtually uninterpretable, being a complex of by chain propagation of dienes proceeds also by incorpo-
13
overlapping multiplets. In Fig. 23 the 25-MHz C spectra
ration of the monomer through one double bond rather
of isotactic (a), atactic (b), and syndiotactic (c) polypropy-
than by 1,4 addition. Thus for butadiene, using coordina-
lene, observed in 20% 1,2,4-trichlorobenzene solution are tion catalysts, these isomeric chains may be formed:
13
shown. (In C NMR it is customary to irradiate the pro-
tons at their resonant frequency to remove the multiplicity
CH 2 CH 2 CH 2
13
1
arising from H- C J-coupling; this increases observing
CH CH CH CH
sensitivity and simplifies the spectrum with no loss of es- CH 2
sential information.) The isotactic and syndiotactic poly- trans-1,4 cis-1,4
mers give very similar spectra but with readily observable
chemical shift differences, especially for the methyl car- CH 2
bons. This sensitivity to stereochemical configuration is CH
particularly clear in the spectrum of the atactic polymer in
CH CH 2 CH CH 2 CH CH 2 CH CH 2
which the methyl resonance is split into peaks correspond-
ing to nine of the ten possible pentad sequences (Table III). CH CH CH
It is also noteworthy that the syndiotactic polymer is less CH 2 CH 2 CH 2
stereoregular than the isotactic and that the configurational ISOTACTIC 1,2 SYNDIOTACTIC 1,2
statistics of both the atactic and syndiotactic polymers de-
part markedly from Bernoullian. This is generally the case Polybutadiene formed by free-radical propagation con-
for chains generated by coordination catalysts. tains all of these units, as we shall see. In Fig. 24 the