Page 66 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 66
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 881
mainly 1,4 (and 1,2) methylene groups. This part of the
spectrum is shown at two values of gain—1X and 5X—
to show the small resonances of the sequences contain-
ing 1,2 units. The major peak (b) corresponds to central
methylenes in cis–cis units; the principal peak (d) is that
of the central 1,4-unit methylene group in trans–trans and
trans–cis units, not discriminated. Peaks (a), (c), (e), and
(m) correspond to sequences involving 1,4 units and one
1,2 unit, while the very small resonances (f ) through (l)
represent sequences containing two 1,2 units. The overall
composition of the polymer is 23% cis-1,4, 58% trans-
1,4, and 19% 1,2. Spectrum (c) is a computer simulation
of (b) based on the assumption of a random distribution of
units in these proportions. The satisfactory fit shows that
free-radical propagation in butadiene polymerization is a
Bernoullian process with regard to the generation of these
isomeric structures.
It has been observed by infrared spectroscopy that
the trans-1,4 content of free-radical polybutadiene or
butadiene–styrene copolymers increases as the polymer-
ization temperature is lowered—from ∼51% at 97 C to
◦
84% at −18 C. Butadiene–styrene copolymers in 75:25
◦
mole ratio are produced commercially in emulsion as SBR
synthetic rubber (see Table II). Most of it is produced at
low temperature, because the higher trans-1,4 content im-
proves its tensile strength and mechanical properties. By
using rhodium salts in aqueous solution very highly stere-
ospecific trans-1,4-polybutadiene can be prepared.
E. Copolymer Structure
A very important structural variable is provided by our
ability to synthesize not only homopolymers with a
single type of monomer unit but also copolymers having
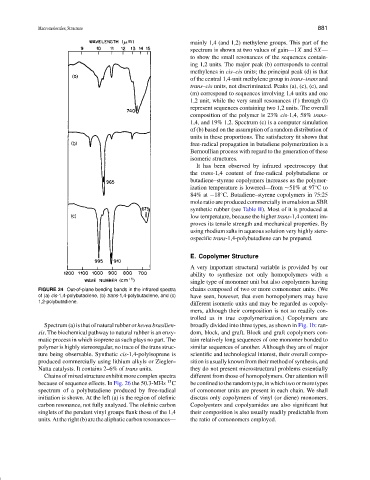
FIGURE 24 Out-of-plane bending bands in the infrared spectra chains composed of two or more comonomer units. (We
of (a) cis-1,4-polybutadiene, (b) trans-1,4-polybutadiene, and (c) have seen, however, that even homopolymers may have
1,2-polybutadiene.
different isomeric units and may be regarded as copoly-
mers, although their composition is not so readily con-
trolled as in true copolymerization.) Copolymers are
Spectrum (a) is that of natural rubber or hevea brasilien- broadly divided into three types, as shown in Fig. 1b: ran-
sis. The biochemical pathway to natural rubber is an enzy- dom, block, and graft. Block and graft copolymers con-
matic process in which isoprene as such plays no part. The tain relatively long sequences of one monomer bonded to
polymer is highly stereoregular, no trace of the trans struc- similar sequences of another. Although they are of major
ture being observable. Synthetic cis-1,4-polyisoprene is scientific and technological interest, their overall compo-
produced commercially using lithium alkyls or Ziegler– sition is usually known from their method of synthesis, and
Natta catalysts. It contains 2–6% of trans units. they do not present microstructural problems essentially
Chains of mixed structure exhibit more complex spectra different from those of homopolymers. Our attention will
13
because of sequence effects. In Fig. 26 the 50.3-MHz C be confined to the random type, in which two or more types
spectrum of a polybutadiene produced by free-radical of comonomer units are present in each chain. We shall
initiation is shown. At the left (a) is the region of olefinic discuss only copolymers of vinyl (or diene) monomers.
carbon resonance, not fully analyzed. The olefinic carbon Copolyesters and copolyamides are also significant but
singlets of the pendant vinyl groups flank those of the 1,4 their composition is also usually readily predictable from
units.Attheright(b)arethealiphaticcarbonresonances— the ratio of comonomers employed.