Page 70 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 70
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 885
methylenes, which from Eq. (62) gives a value of 3.31 for More searching tests of the propagation mechanism can
r 1 . Evaluation of the methylene resonance of be obtained from consideration of tetrad intensities. By
such analysis it is found that the relative reactivity of a
CH 3 CH 3
growing free radical ending in vinylidene chloride de-
C CH 2 C pends on whether the penultimate unit is another vinyli-
dene chloride unit or an isobutylene unit; if the latter, the
CH 3 CH 3
chain is twice as likely to add vinylidene chloride.
cannot be readily carried out because of overlaps, even A system of much interest is the copolymerization of
though it would most directly provide a value ofr 2 . Instead ethylene and propylene. This reaction requires coordina-
we note that tion catalysts rather than free-radical initiators. With in-
soluble coordination catalysts such as vanadium trichlo-
[m 1 m 2 ](+[m 2 m 1 ]) = 2F 2 (1 − P 22 ) (64)
ride, together with aluminum alkyls or chloroaluminum
or alkyls, the heterogeneous propagation process is too com-
plex for the simple treatment we have discussed. How-
2F 2 r 2 f 2
[m 1 m 2 ](+[m 2 m 1 ]) = 2F 2 − . (65) ever, with soluble vanadium compounds—VOCl 3 , VCl 4 ,
1 − f 2 (1 − r 2 )
or vanadium triacetylacetonate—the reaction takes place
From the group of resonances near 2.8 ppm, a value of in a homogeneous phase and can be treated within our
[m 1 m 2 ] of 0.56 is obtained, from which a value of r 2 of framework, although we shall not enter into details. In
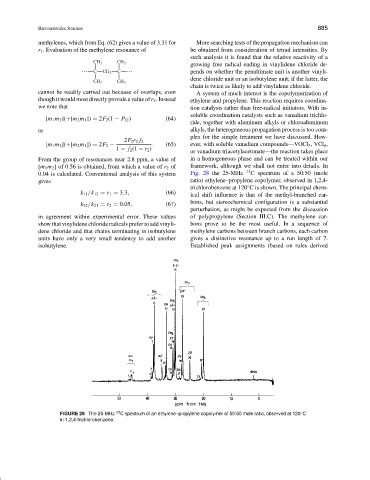
0.04 is calculated. Conventional analysis of this system Fig. 28 the 25-MHz 13 C spectrum of a 50:50 (mole
gives ratio) ethylene–propylene copolymer, observed in 1,2,4-
◦
trichlorobenzene at 120 C is shown. The principal chem-
k 11 /k 12 = r 1 = 3.3, (66) ical shift influence is that of the methyl-branched car-
bons, but stereochemical configuration is a substantial
k 22 /k 21 = r 2 = 0.05, (67)
perturbation, as might be expected from the discussion
in agreement within experimental error. These values of polypropylene (Section III.C). The methylene car-
show that vinylidene chloride radicals prefer to add vinyli- bons prove to be the most useful. In a sequence of
dene chloride and that chains terminating in isobutylene methylene carbons between branch carbons, each carbon
units have only a very small tendency to add another gives a distinctive resonance up to a run length of 7.
isobutylene. Established peak assignments (based on rules derived
13
FIGURE 28 The 25-MHz C spectrum of an ethylene–propylene copolymer of 50:50 mole ratio, observed at 120 C
◦
in 1,2,4-trichlorobenzene.