Page 73 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 73
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
888 Macromolecules, Structure
crosslinks. This is also true of course of homopolymers of four carbons or more removed from a branch or chain
such monomers: end: It constitutes about 80% of the spectral intensity. The
C 1 carbons (i.e., methyl groups) and C 2 carbons are the
CH CH 2 most shielded, branch point carbons the least. Main-chain
CH 2 CH 2 CH 2
RCH CH 2 CH CH carbons β to the branch are more shielded while those α to
the branch are less shielded than unperturbed methylenes.
R
The composition of this polyethylene is shown in Table IV.
CH CH 2
The predominant branch type is n-butyl. Both amyl and
P-DIVINYL BENZENE
CH CH 2 butylbranchesarebelievedtobeformedbyintramolecular
chain transfer or “backbiting”:
Here, we discuss branching introduced by processes that
are under less specific control and involve chain transfer
reaction of various types. CH 2 CH 2 CH CH 3
Branches are of particular importance in polyethylene (CH 2 ) n (CH 2 ) n
(see Fig. 2), as their presence reduces the melting point
and extent of crystallinity (Section III.A). High-pressure This reaction is evidently most probable when n = 3 or 4,
polyethylene is found by infrared spectroscopy to have has a low but finite probability when n = 1, and has zero
unusually large numbers of methyl groups, normally ex- probability when n = 0 or 2.
pected only at chain ends (Section III.A). When combined The complex appearance of the ethyl branch methyl
with molecular weight measurements, these results indi- resonance at ∼11.0 ppm suggests that these branches may
cate that there are many more ends than molecules—or occur in groups or with some similar complication. The
in other words that the chains contain branches. Carbon- branches described in Table IV as “hexyl or longer” are
13 NMR can supply details of the types and distribu- believed to be truly long, as illustrated in Fig. 2 (and
13
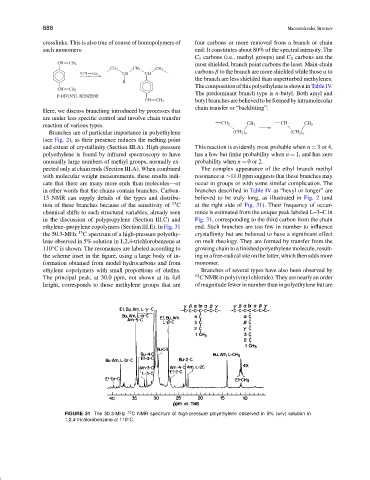
tion of these branches because of the sensitivity of C at the right side of Fig. 31). Their frequency of occur-
chemical shifts to such structural variables, already seen rence is estimated from the unique peak labeled L–3–C in
in the discussion of polypropylene (Section III.C) and Fig. 31, corresponding to the third carbon from the chain
ethylene–propylene copolymers (Section III.E). In Fig. 31 end. Such branches are too few in number to influence
13
the 50.3-MHz C spectrum of a high-pressure polyethy- crystallinity but are believed to have a significant effect
lene observed in 5% solution in 1,2,4-trichlorobenzene at on melt rheology. They are formed by transfer from the
◦
110 C is shown. The resonances are labeled according to growing chain to a finished polyethylene molecule, result-
the scheme inset in the figure, using a large body of in- ing in a free-radical site on the latter, which then adds more
formation obtained from model hydrocarbons and from monomer.
ethylene copolymers with small proportions of olefins. Branches of several types have also been observed by
The principal peak, at 30.0 ppm, not shown at its full 13 C NMR in poly(vinyl chloride). They are nearly an order
height, corresponds to those methylene groups that are of magnitude fewer in number than in polyethylene but are
FIGURE 31 The 50.3-MHz 13 C NMR spectrum of high-pressure polyethylene observed in 5% (w/v) solution in
1,2,4-trichlorobenzene at 110 C.
◦