Page 75 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 75
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
890 Macromolecules, Structure
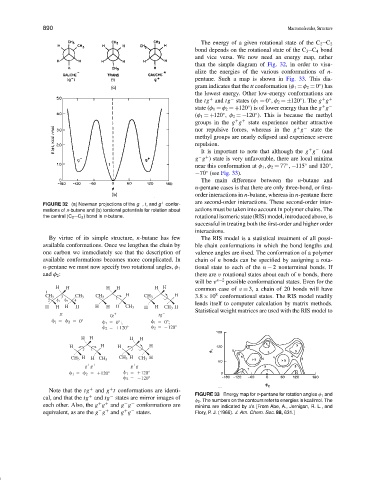
The energy of a given rotational state of the C 2 C 2
bond depends on the rotational state of the C 3 C 4 bond
and vice versa. We now need an energy map, rather
than the simple diagram of Fig. 32, in order to visu-
alize the energies of the various conformations of n-
pentane. Such a map is shown in Fig. 33. This dia-
gram indicates that the tt conformation (φ 1 = φ 2 = 0 ) has
◦
the lowest energy. Other low-energy conformations are
+ −
the tg and tg states (φ 1 = 0 , φ 2 = ±120 ). The g g
◦
+ +
◦
◦
state (φ 1 = φ 2 = +120 ) is of lower energy than the g g
+ −
(φ 1 = +120 , φ 2 = −120 ). This is because the methyl
◦
◦
+ +
groups in the g g state experience neither attractive
+ −
nor repulsive forces, whereas in the g g state the
methyl groups are nearly eclipsed and experience severe
repulsion.
+ −
It is important to note that although the g g (and
− +
g g ) state is very unfavorable, there are local minima
◦
◦
◦
near this conformation at φ 1 ,φ 2 = 77 , −115 and 120 ,
◦
−70 (see Fig. 33).
The main difference between the n-butane and
n-pentane cases is that there are only three-bond, or first-
order interactions in n-butane, whereas in n-pentane there
+ are second-order interactions. These second-order inter-
−
FIGURE 32 (a) Newman projections of the g , t, and g confor-
mations of n-butane and (b) torsional potentials for rotation about actions must be taken into account in polymer chains. The
the central (C 2 C 3 ) bond in n-butane. rotational isomeric state (RIS) model, introduced above, is
successful in treating both the first-order and higher order
interactions.
By virtue of its simple structure, n-butane has few The RIS model is a statistical treatment of all possi-
available conformations. Once we lengthen the chain by ble chain conformations in which the bond lengths and
one carbon we immediately see that the description of valence angles are fixed. The conformation of a polymer
available conformations becomes more complicated. In chain of n bonds can be specified by assigning a rota-
tional state to each of the n − 2 nonterminal bonds. If
n-pentane we must now specify two rotational angles, φ 1
and φ 2 : there are v rotational states about each of n bonds, there
will be v n−2 possible conformational states. Even for the
H H H H H H
1 common case of v = 3, a chain of 20 bonds will have
8
CH 3 3 CH 3 CH 3 H CH 3 H 3.8 × 10 conformational states. The RIS model readily
2 1 2 4
H H H H H H H CH 3 H H CH 3 H lends itself to computer calculation by matrix methods.
tt tg tg Statistical weight matrices are used with the RIS model to
1 2 0
1 0
; 1 0
;
2 120
2 120
H H H H
H H H H
H H H
CH 3 H CH 3 CH 3 CH 3
g g g g
1 2 120
1 120
2 120
Note that the tg + and g t conformations are identi-
+
+
−
cal, and that the tg and tg states are mirror images of FIGURE 33 Energy map for n-pentane for rotation angles φ 1 and
φ 2 . The numbers on the contours refer to energies in kcal/mol. The
+ +
− −
each other. Also, the g g and g g conformations are minima are indicated by x’s [From Abe, A., Jernigan, R. L., and
+ −
− +
equivalent, as are the g g and g g states. Flory, P. J. (1966). J. Am. Chem. Soc. 88, 631.]