Page 80 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 80
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 895
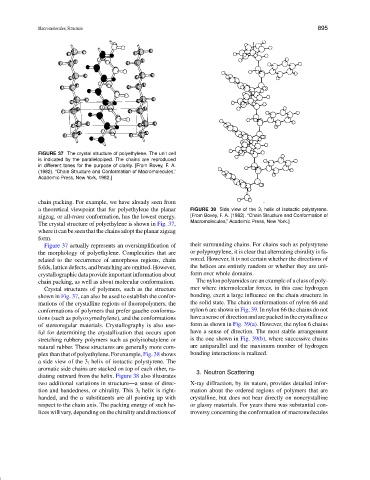
FIGURE 37 The crystal structure of polyethylene. The unit cell
is indicated by the parallelopiped. The chains are reproduced
in different tones for the purpose of clarity. [From Bovey, F. A.
(1982). “Chain Structure and Conformation of Macromolecules,”
Academic Press, New York, 1982.]
chain packing. For example, we have already seen from
a theoretical viewpoint that for polyethylene the planar FIGURE 38 Side view of the 3 l helix of isotactic polystyrene.
zigzag, or all-trans conformation, has the lowest energy. [From Bovey, F. A. (1982). “Chain Structure and Conformation of
The crystal structure of polyethylene is shown in Fig. 37, Macromolecules,” Academic Press, New York.]
where it can be seen that the chains adopt the planar zigzag
form.
Figure 37 actually represents an oversimplification of their surrounding chains. For chains such as polystyrene
the morphology of polyethylene. Complexities that are or polypropylene, it is clear that alternating chirality is fa-
related to the occurrence of amorphous regions, chain vored. However, it is not certain whether the directions of
folds, lattice defects, and branching are omitted. However, the helices are entirely random or whether they are uni-
crystallographic data provide important information about form over whole domains.
chain packing, as well as about molecular conformation. The nylon polyamides are an example of a class of poly-
Crystal structures of polymers, such as the structure mer where intermolecular forces, in this case hydrogen
shown in Fig. 37, can also be used to establish the confor- bonding, exert a large influence on the chain structure in
mations of the crystalline regions of fluoropolymers, the the solid state. The chain conformations of nylon 66 and
conformations of polymers that prefer gauche conforma- nylon 6 are shown in Fig. 39. In nylon 66 the chains do not
tions (such as polyoxymethylene), and the conformations have a sense of direction and are packed in the crystalline α
of stereoregular materials. Crystallography is also use- form as shown in Fig. 39(a). However, the nylon 6 chains
ful for determining the crystallization that occurs upon have a sense of direction. The most stable arrangement
stretching rubbery polymers such as polyisobutylene or is the one shown in Fig. 39(b), where successive chains
natural rubber. These structures are generally more com- are antiparallel and the maximum number of hydrogen
plex than that of polyethylene. For example, Fig. 38 shows bonding interactions is realized.
a side view of the 3 l helix of isotactic polystyrene. The
aromatic side chains are stacked on top of each other, ra-
3. Neutron Scattering
diating outward from the helix. Figure 38 also illustrates
two additional variations in structure—a sense of direc- X-ray diffraction, by its nature, provides detailed infor-
tion and handedness, or chirality. This 3 l helix is right- mation about the ordered regions of polymers that are
handed, and the α substituents are all pointing up with crystalline, but does not bear directly on noncrystalline
respect to the chain axis. The packing energy of such he- or glassy materials. For years there was substantial con-
lices will vary, depending on the chirality and directions of troversy concerning the conformation of macromolecules