Page 65 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 65
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
880 Macromolecules, Structure
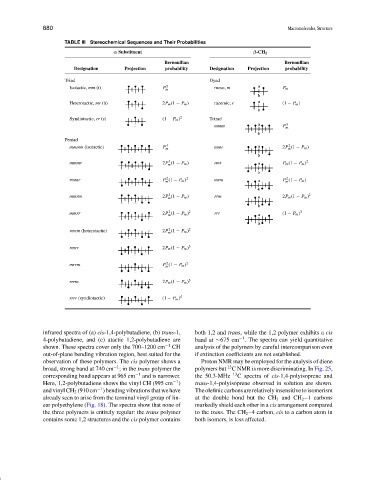
TABLE III Stereochemical Sequences and Their Probabilities
α Substituent β-CH 2
Bernoullian Bernoullian
Designation Projection probability Designation Projection probability
Triad Dyad
Isotactic, mm (i) P m 2 meso, m P m
Heterotactic, mr (h) 2P m (1 − P m ) racemic, r (1 − P m )
Syndiotactic, rr (s) (1 − P m ) 2 Tetrad
mmm P m 3
Pentad
2
mmmm (isotactic) P m 4 mmr 2P (1 − P m )
m
3
mmmr 2P (1 − P m ) rmr P m (1 − P m ) 2
m
2
2
rmmr P (1 − P m ) 2 mrm P (1 − P m )
m
m
3
mmrm 2P (1 − P m ) rrm 2P m (1 − P m ) 2
m
2
mmrr 2P (1 − P m ) 2 rrr (1 − P m ) 3
m
2
rmrm (heterotactic) 2P (1 − P m ) 2
m
rmrr 2P m (1 − P m ) 3
2
mrrm P (1 − P m ) 2
m
rrrm 2P m (1 − P m ) 3
rrrr (syndiotactic) (1 − P m ) 4
infrared spectra of (a) cis-1,4-polybutadiene, (b) trans-1, both 1,2 and trans, while the 1,2 polymer exhibits a cis
−1
4-polybutadiene, and (c) atactic 1,2-polybutadiene are band at ∼675 cm . The spectra can yield quantitative
shown. These spectra cover only the 700–1200 cm −1 CH analysis of the polymers by careful intercomparison even
out-of-plane bending vibration region, best suited for the if extinction coefficients are not established.
observation of these polymers. The cis polymer shows a Proton NMR may be employed for the analysis of diene
13
−1
broad, strong band at 740 cm ; in the trans polymer the polymers but C NMR is more discriminating. In Fig. 25,
corresponding band appears at 965 cm −1 and is narrower. the 50.3-MHz 13 C spectra of cis-1,4-polyisoprene and
−1
Here, 1,2-polybutadiene shows the vinyl CH (995 cm ) trans-1,4-polyisoprene observed in solution are shown.
−1
and vinyl CH 2 (910 cm ) bending vibrations that we have Theolefiniccarbonsarerelativelyinsensitivetoisomerism
already seen to arise from the terminal vinyl group of lin- at the double bond but the CH 3 and CH 2 1 carbons
ear polyethylene (Fig. 18). The spectra show that none of markedly shield each other in a cis arrangement compared
the three polymers is entirely regular: the trans polymer to the trans. The CH 2 4 carbon, cis to a carbon atom in
contains some 1,2 structures and the cis polymer contains both isomers, is less affected.