Page 194 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 194
P1: GSS Final Pages
Encyclopedia of Physical Science and Technology EN009F-398 July 6, 2001 20:34
Main Group Elements 3
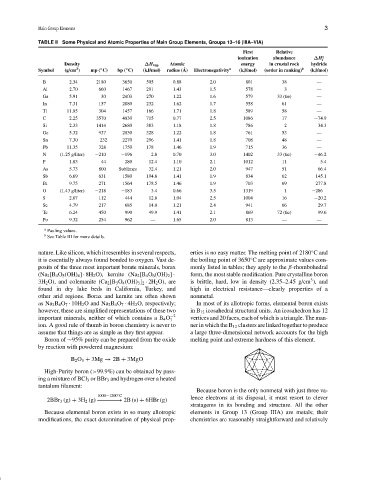
TABLE II Some Physical and Atomic Properties of Main Group Elements, Groups 13–16 (IIIA–VIA)
First Relative
ionization abundance ∆H ◦
f
Density ∆H vap Atomic energy in crustal rock hydride
3
Symbol (g/cm ) mp ( C) bp ( C) (kJ/mol) radius ( ˚ A) Electronegativity a (kJ/mol) (order in ranking) b (kJ/mol)
◦
◦
B 2.34 2180 3650 505 0.88 2.0 801 38 —
Al 2.70 660 1467 291 1.43 1.5 578 3 —
Ga 5.91 30 2403 270 1.22 1.6 579 33 (tie) —
In 7.31 157 2080 232 1.62 1.7 558 61 —
Tl 11.85 304 1457 166 1.71 1.8 589 58 —
C 2.25 3570 4830 715 0.77 2.5 1086 17 −74.9
Si 2.33 1414 2680 383 1.18 1.8 786 2 34.3
Ge 5.32 937 2830 328 1.22 1.8 761 53 —
Sn 7.30 232 2270 296 1.41 1.8 708 48 —
Pb 11.35 328 1750 178 1.46 1.9 715 36 —
N (1.25 g/liter) −210 −196 2.8 0.70 3.0 1402 33 (tie) −46.2
P 1.83 44 280 12.4 1.10 2.1 1012 11 5.4
As 5.73 800 Sublimes 32.4 1.21 2.0 947 51 66.4
Sb 6.69 631 1580 194.8 1.41 1.9 834 62 145.1
Bi 9.75 271 1564 178.5 1.46 1.9 703 69 277.8
O (1.43 g/liter) −218 −183 3.4 0.66 3.5 1319 1 −286
S 2.07 112 444 12.6 1.04 2.5 1004 16 −20.2
Se 4.79 217 685 14.0 1.21 2.4 941 66 29.7
Te 6.24 450 990 49.9 1.41 2.1 869 72 (tie) 99.6
Po 9.32 254 962 — 1.65 2.0 813 — —
a Pauling values.
b See Table III for more details.
◦
nature. Like silicon, which it resembles in several respects, erties is no easy matter. The melting point of 2180 C and
◦
it is essentially always found bonded to oxygen. Vast de- the boiling point of 3650 C are approximate values com-
posits of the three most important borate minerals, borax monly listed in tables; they apply to the β-rhombohedral
(Na 2 [B 4 O 5 (OH) 4 ] · 8H 2 O), kernite (Na 2 [B 4 O 6 (OH) 2 ] · form, the most stable modification. Pure crystalline boron
3
3H 2 O), and colemanite (Ca 2 [B 3 O 4 (OH) 3 ] 2 · 2H 2 O), are is brittle, hard, low in density (2.35–2.45 g/cm ), and
found in dry lake beds in California, Turkey, and high in electrical resistance—clearly properties of a
other arid regions. Borax and kernite are often shown nonmetal.
as Na 2 B 4 O 7 · 10H 2 O and Na 2 B 4 O 7 · 4H 2 O, respectively; In most of its allotropic forms, elemental boron exists
however, these are simplified representations of these two in B 12 icosahedral structural units. An icosahedron has 12
−2
important minerals, neither of which contains a B 4 O 7 vertices and 20 faces, each of which is a triangle. The man-
ion. A good rule of thumb in boron chemistry is never to ner in which the B 12 clusters are linked together to produce
assume that things are as simple as they first appear. a large three-dimensional network accounts for the high
Boron of ∼95% purity can be prepared from the oxide melting point and extreme hardness of this element.
by reaction with powdered magnesium:
B 2 O 3 + 3Mg → 2B + 3MgO
High-Purity boron (>99.9%) can be obtained by pass-
ing a mixture of BCl 3 or BBr 3 and hydrogen over a heated
tantalum filament:
Because boron is the only nonmetal with just three va-
◦
1000−1200 C
2BBr 3 (g) + 3H 2 (g) −−−−−−→ 2B (s) + 6HBr (g) lence electrons at its disposal, it must resort to clever
stratagems in its bonding and structure. All the other
Because elemental boron exists in so many allotropic elements in Group 13 (Group IIIA) are metals; their
modifications, the exact determination of physical prop- chemistries are reasonably straightforward and relatively