Page 225 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 225
P1: GPB Final Pages
Encyclopedia of Physical Science and Technology EN009I-420 July 10, 2001 15:8
372 Mesoporous Materials, Synthesis and Properties
supported by a body of evidence. The following section
attempts to explain the prevailing mechanisms and the un-
derlying factors that determine mesophase formation.
There are two general methods used to prepare meso-
porous molecular sieves:
1. Assembly of dissolved inorganic species around
surfactant arrays via liquid crystal templating (LCT)
mechanisms
2. Intercalation of surfactant ions into layered silicates
via a folded sheet mechanism (FSM materials).
1. Liquid Crystal Templating (LCT) Mechanisms
The key aspect of the LCT mechanism is that the liq-
uid crystalline mesophases or micelles act as templates
rather than individual single molecules or ions. Accord-
ingly, the final product is an inorganic (e.g., silicate) skele-
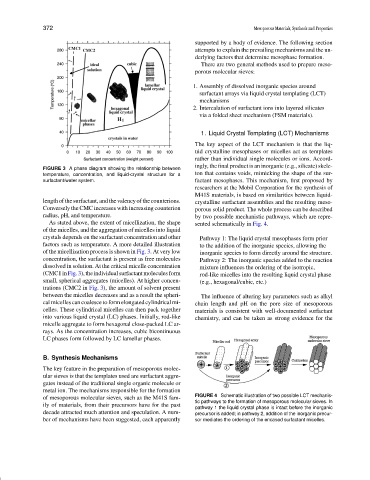
FIGURE 3 A phase diagram showing the relationship between
temperature, concentration, and liquid-crystal structure for a ton that contains voids, mimicking the shape of the sur-
surfactant/water system. factant mesophases. This mechanism, first proposed by
researchers at the Mobil Corporation for the synthesis of
M41S materials, is based on similarities between liquid-
length of the surfactant, and the valency of the counterions. crystalline surfactant assemblies and the resulting meso-
Conversely the CMC increases with increasing counterion porous solid product. The whole process can be described
radius, pH, and temperature. by two possible mechanistic pathways, which are repre-
As stated above, the extent of micellization, the shape sented schematically in Fig. 4.
of the micelles, and the aggregation of micelles into liquid
crystals depends on the surfactant concentration and other Pathway 1: The liquid crystal mesophases form prior
factors such as temperature. A more detailed illustration to the addition of the inorganic species, allowing the
of the micellization process is shown in Fig. 3. At very low inorganic species to form directly around the structure.
concentration, the surfactant is present as free molecules Pathway 2: The inorganic species added to the reaction
dissolved in solution. At the critical micelle concentration mixture influences the ordering of the isotropic,
(CMC1inFig.3),theindividualsurfactantmoleculesform rod-like micelles into the resulting liquid crystal phase
small, spherical aggregates (micelles). At higher concen- (e.g., hexagonal/cubic, etc.)
trations (CMC2 in Fig. 3), the amount of solvent present
between the micelles decreases and as a result the spheri- The influence of altering key parameters such as alkyl
cal micelles can coalesce to form elongated cylindrical mi- chain length and pH on the pore size of mesoporous
celles. These cylindrical micelles can then pack together materials is consistent with well-documented surfactant
into various liquid crystal (LC) phases. Initially, rod-like chemistry, and can be taken as strong evidence for the
micelle aggregate to form hexagonal close-packed LC ar-
rays. As the concentration increases, cubic bicontinuous
LC phases form followed by LC lamellar phases.
B. Synthesis Mechanisms
The key feature in the preparation of mesoporous molec-
ular sieves is that the templates used are surfactant aggre-
gates instead of the traditional single organic molecule or
metal ion. The mechanisms responsible for the formation
of mesoporous molecular sieves, such as the M41S fam- FIGURE 4 Schematic illustration of two possible LCT mechanis-
tic pathways to the formation of mesoporous molecular sieves. In
ily of materials, from their precursors have for the past
pathway 1 the liquid crystal phase is intact before the inorganic
decade attracted much attention and speculation. A num- precursor is added; in pathway 2, addition of the inorganic precur-
ber of mechanisms have been suggested, each apparently sor mediates the ordering of the encased surfactant micelles.