Page 24 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 24
P1: FYK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-11 May 7, 2001 12:19
232 Actinide Elements
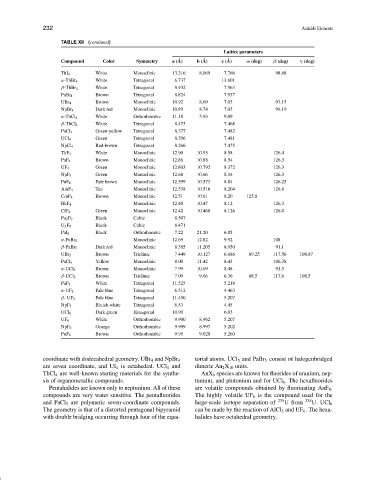
TABLE XII (continued)
Lattice parameters
Compound Color Symmetry a ( ˚ A) b ( ˚ A) c ( ˚ A) α (deg) β (deg) γ (deg)
ThI 4 White Monoclinic 13.216 8.069 7.766 98.68
α-ThBr 4 White Tetragonal 6.737 13.601
β-ThBr 4 White Tetragonal 8.932 7.963
PaBr 4 Brown Tetragonal 8.824 7.957
UBr 4 Brown Monoclinic 10.92 8.69 7.05 93.15
NpBr 4 Dark red Monoclinic 10.89 8.74 7.05 94.19
α-ThCl 4 White Orthorhombic 11.18 5.93 9.09
β-ThCl 4 White Tetragonal 8.473 7.468
PaCl 4 Green-yellow Tetragonal 8.377 7.482
UCl 4 Green Tetragonal 8.296 7.481
NpCl 4 Red-brown Tetragonal 8.266 7.475
ThF 4 White Monoclinic 12.90 10.93 8.58 126.4
Brown Monoclinic 12.86 10.88 8.54 126.3
PaF 4
UF 4 Green Monoclinic 12.803 10.792 8.372 126.3
NpF 4 Green Monoclinic 12.68 10.66 8.34 126.3
PuF 4 Pale brown Monoclinic 12.599 10.573 8.84 126.25
AmF 4 Tan Monoclinic 12.538 10.516 8.204 126.8
CmF 4 Brown Monoclinic 12.51 10.61 8.20 125.8
BkF 4 Monoclinic 12.40 10.47 8.12 126.3
CfF 4 Green Monoclinic 12.42 10.468 8.126 126.0
Pa 2 F 9 Black Cubic 8.507
U 2 F 9 Black Cubic 8.471
PaI 5 Black Orthorhombic 7.22 21.20 6.85
α-PaBr 5 Monoclinic 12.69 12.82 9.92 108
β-PaBr 5 Dark red Monoclinic 8.385 11.205 8.950 91.1
UBr 5 Brown Triclinic 7.449 10.127 6.686 89.25 117.56 108.87
PaCl 5 Yellow Monoclinic 8.00 11.42 8.43 106.38
α-UCl 5 Brown Monoclinic 7.99 10.69 8.48 91.5
β-UCl 5 Brown Triclinic 7.09 9.66 6.36 88.5 117.6 108.5
PaF 5 White Tetragonal 11.525 5.218
α-UF 5 Pale blue Tetragonal 6.512 4.463
β-UF 5 Pale blue Tetragonal 11.450 5.207
NpF 5 Bluish-white Tetragonal 6.53 4.45
UCl 6 Dark green Hexagonal 10.90 6.03
UF 6 White Orthorhombic 9.900 8.962 5.207
NpF 6 Orange Orthorhombic 9.909 8.997 5.202
PuF 6 Brown Orthorhombic 9.95 9.020 5.260
torial atoms. UCl 5 and PaBr 5 consist of halogenbridged
coordinate with dodecahedral geometry. UBr 4 and NpBr 4
are seven coordinate, and UI 4 is octahedral. UCl 4 and dimeric An 2 X 10 units.
ThCl 4 are well-known starting materials for the synthe- AnX 6 species are known for fluorides of uranium, nep-
sis of organometallic compounds. tunium, and plutonium and for UCl 6 . The hexafluorides
Pentahalides are known only to neptunium. All of these are volatile compounds obtained by fluorinating AnF 4 .
compounds are very water sensitive. The pentafluorides The highly volatile UF 6 is the compound used for the
and PaCl 5 are polymeric seven-coordinate compounds. large-scale isotope separation of 235 U from 238 U. UCl 6
The geometry is that of a distorted pentagonal bipyramid can be made by the reaction of AlCl 3 and UF 6 . The hexa-
with double bridging occurring through four of the equa- halides have octahedral geometry.