Page 25 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 25
P1: FYK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-11 May 7, 2001 12:19
Actinide Elements 233
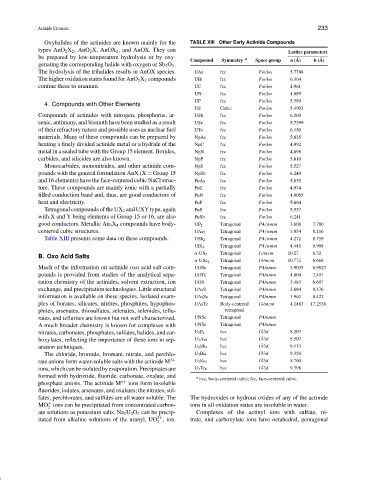
Oxyhalides of the actinides are known mainly for the TABLE XIII Other Early Actinide Compounds
types AnO 2 X 2 , AnO 2 X, AnOX 2 , and AnOX. They can
Lattice parameters
be prepared by low-temperature hydrolysis or by oxy-
Compound Symmetry a Space group a ( ˚ A) b ( ˚ A)
genating the corresponding halide with oxygen or Sb 2 O 3 .
The hydrolysis of the trihalides results in AnOX species. UAs fcc Fm3m 5.7788
The higher oxidation states found for AnO 2 X 2 compounds UBi fcc Fm3m 6.364
confine these to uranium. UC fcc Fm3m 4.961
UN fcc Fm3m 4.889
UP fcc Fm3m 5.589
4. Compounds with Other Elements
US Cubic Pm3m 5.4903
Compounds of actinides with nitrogen, phosphorus, ar- USb fcc Fm3m 6.203
senic, antimony, and bismuth have been studied as a result USe fcc Fm3m 5.7399
of their refractory nature and possible uses as nuclear fuel UTe fcc Fm3m 6.150
materials. Many of these compounds can be prepared by NpAs fcc Fm3m 5.835
heating a finely divided actinide metal or a hydride of the NpC fcc Fm3m 4.992
metal in a sealed tube with the Group 15 element. Borides, NpN fcc Fm3m 4.898
carbides, and silicides are also known. NpP fcc Fm3m 5.610
Monocarbides, mononitrides, and other actinide com- NpS fcc Fm3m 5.527
pounds with the general formulation AnX (X = Group 15 NpSb fcc Fm3m 6.249
and 16 elements) have the face-centered cubic NaCl struc- PuAs fcc Fm3m 5.855
ture. These compounds are mainly ionic with a partially PuC fcc Fm3m 4.974
filled conduction band and, thus, are good conductors of PuN fcc Fm3m 4.9055
heat and electricity. PuP fcc Fm3m 5.664
Tetragonal compounds of the UX 2 and UXY type, again PuS fcc Fm3m 5.537
with X and Y being elements of Group 15 or 16, are also PuSb fcc Fm3m 6.241
good conductors. Metallic An 3 X 4 compounds have body- UP 2 Tetragonal P4/nmm 3.808 7.780
centered cubic structures. UAs 2 Tetragonal P4/nmm 3.954 8.116
Table XIII presents some data on these compounds. USb 2 Tetragonal P4/nmm 4.272 8.759
UBi 2 Tetragonal P4/nmm 4.445 8.908
α-US 2 Tetragonal I 1 /mcm 10.27 6.32
B. Oxo Acid Salts
α-USe 2 Tetragonal I4/mcm 10.772 6.668
Much of the information on actinide oxo acid salt com- UOSe Tetragonal P4/nmm 3.9035 6.9823
pounds is provided from studies of the analytical sepa- UOTe Tetragonal P4/nmm 4.004 7.491
ration chemistry of the actinides, solvent extraction, ion UOS Tetragonal P4/nmm 3.483 6.697
exchange, and precipitation technologies. Little structural UAsS Tetragonal P4/nmm 3.884 8.176
information is available on these species. Isolated exam- UAsSe Tetragonal P4/nmm 3.962 8.422
ples of borates, silicates, nitrites, phosphites, hypophos- UAsTe Body-centered I4/mcm 4.1483 17.2538
phites, arsenates, thiosulfates, selenates, selenides, tellu- tetragonal
rates, and tellurites are known but not well characterized. UNSe Tetragonal P4/nmm
A much broader chemistry is known for complexes with UNTe Tetragonal P4/nmm
nitrates, carbonates, phosphates, sulfates, halides, and car- U 3 P 4 bcc I ¯ 43d 8.207
boxylates, reflecting the importance of these ions in sep- U 3 As 4 bcc I ¯ 43d 8.507
aration techniques. U 3 Sb 4 bcc I ¯ 43d 9.113
The chloride, bromide, bromate, nitrate, and perchlo- U 3 Bi 4 bcc I ¯ 43d 9.350
rate anions form water-soluble salts with the actinide M 3+ U 3 Se 4 bcc I ¯ 43d 8.760
ions,whichcanbeisolatedbyevaporation.Precipitatesare U 3 Te 4 bcc I ¯ 43d 9.398
formed with hydroxide, fluoride, carbonate, oxalate, and a bcc, body-centered cubic; fcc, face-centered cubic.
phosphate anions. The actinide M 4+ ions form insoluble
fluorides, iodates, arsenates, and oxalates; the nitrates, sul-
fates, perchlorates, and sulfides are all water soluble. The The hydroxides or hydrous oxides of any of the actinide
MO ions can be precipitated from concentrated carbon- ions in all oxidation states are insoluble in water.
+
2
ate solutions as potassium salts. Na 2 U 2 O 7 can be precip- Complexes of the actinyl ions with sulfate, ni-
itated from alkaline solutions of the uranyl, UO , ion. trate, and carboxylate ions have octahedral, pentagonal
2+
2