Page 38 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 38
P1: ZBU Final Pages
Encyclopedia of Physical Science and Technology EN002F-55 May 22, 2001 21:6
126 Bioinorganic Chemistry
molecules, where large structural changes, such as bond
breaking, usually are coupled to the transfer of electrons.
By placing an inorganic atom or cluster of atoms within
a protein and tuning the redox properties with the sur-
rounding protein environment, nature can transfer elec-
trons from one location to another within the cell. Further-
more, proteins can set up coupled pathways that enable the
facile exchange of electrons from one redox center to an-
other over relatively long distances. Three general types
of inorganic sites are used in biology to transfer electrons:
hemes, iron–sulfur clusters, and blue copper centers. Each
of these will be described below.
The heme proteins that are involved in electron transfer
are denoted cytochromes and the best studied of these are
the cytochrome c’s. Cytochromes are highly water solu-
ble, have relatively low molecular weight (∼10 kDa), are
highly stable, and are easily purified. Cytochromes are in-
volved solely in the electron transfer cycle between the +2
and +3 oxidation states of iron. The range of reduction po-
tentials for cytochromes is between −100 and +400 mV
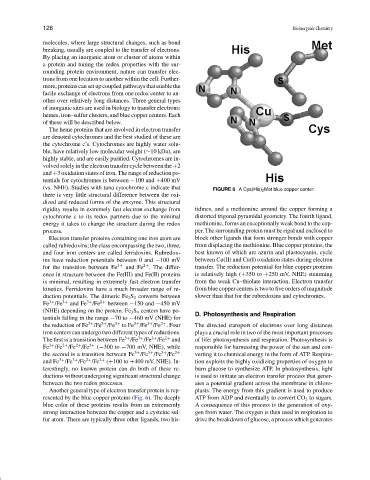
(vs. NHE). Studies with tuna cytochrome c indicate that FIGURE 6 A Cys(His) 2 Met blue copper center.
there is very little structural difference between the oxi-
dized and reduced forms of the enzyme. This structural
rigidity results in extremely fast electron exchange from tidines, and a methionine around the copper forming a
cytochrome c to its redox partners due to the minimal distorted trigonal pyramidal geometry. The fourth ligand,
energy it takes to change the structure during the redox methionine, forms an exceptionally weak bond to the cop-
process. per. The surrounding protein must be rigid and enclosed to
Electron transfer proteins containing one iron atom are block other ligands that form stronger bonds with copper
called rubredoxins; the class encompassing the two, three, from displacing the methionine. Blue copper proteins, the
and four iron centers are called ferridoxins. Rubredox- best known of which are azurin and plastocyanin, cycle
ins have reduction potentials between 0 and −100 mV between Cu(II) and Cu(I) oxidation states during electron
2+
for the transition between Fe 3+ and Fe . The differ- transfer. The reduction potential for blue copper proteins
ence in structure between the Fe(III) and Fe(II) proteins is relatively high (+350 to +250 mV, NHE) stemming
is minimal, resulting in extremely fast electron transfer from the weak Cu–thiolate interaction. Electron transfer
kinetics. Ferridoxins have a much broader range of re- from blue copper centers is two to five orders of magnitude
duction potentials. The dimeric Fe 2 S 2 converts between slower than that for the rubredoxins and cytochromes.
3+
Fe /Fe 3+ and Fe /Fe 2+ between −150 and −450 mV
3+
(NHE) depending on the protein. Fe 3 S 4 centers have po-
D. Photosynthesis and Respiration
tentials falling in the range −70 to −460 mV (NHE) for
3+
3+
3+
3+
2+
the reduction of Fe /Fe /Fe 3+ to Fe /Fe /Fe . Four The directed transport of electrons over long distances
iron centers can undergo two different types of reductions. plays a crucial role in two of the most important processes
The first is a transition between Fe /Fe /Fe /Fe 2+ and of life: photosynthesis and respiration. Photosynthesis is
2+
3+
3+
2+
2+
3+
Fe /Fe /Fe /Fe 2+ (−300 to −700 mV, NHE), while responsible for harnessing the power of the sun and con-
3+
3+
3+
the second is a transition between Fe /Fe /Fe /Fe 2+ verting it to chemical energy in the form of ATP. Respira-
and Fe /Fe /Fe /Fe 2+ (+100 to +400 mV, NHE). In- tion exploits the highly oxidizing properties of oxygen to
3+
3+
2+
terestingly, no known protein can do both of these re- burn glucose to synthesize ATP. In photosynthesis, light
ductions without undergoing significant structural change is used to initiate an electron transfer process that gener-
between the two redox processes. ates a potential gradient across the membrane in chloro-
Another general type of electron transfer protein is rep- plasts. The energy from this gradient is used to produce
resented by the blue copper proteins (Fig. 6). The deeply ATP from ADP and eventually to convert CO 2 to sugars.
blue color of these proteins results from an extrememly A consequence of this process is the generation of oxy-
strong interaction between the copper and a cysteine sul- gen from water. The oxygen is then used in respiration to
fur atom. There are typically three other ligands, two his- drive the breakdown of glucose, a process which generates