Page 42 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 42
P1: ZBU Final Pages
Encyclopedia of Physical Science and Technology EN002F-55 May 22, 2001 21:6
130 Bioinorganic Chemistry
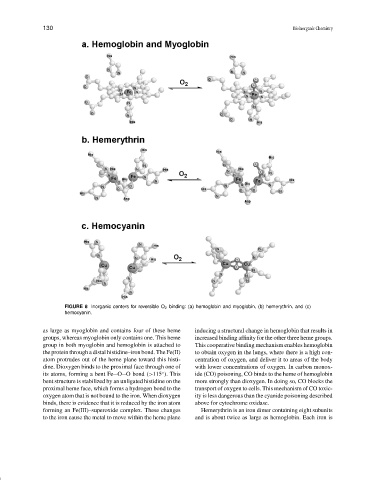
FIGURE 8 Inorganic centers for reversible O 2 binding: (a) hemoglobin and myoglobin, (b) hemerythrin, and (c)
hemocyanin.
as large as myoglobin and contains four of these heme inducing a structural change in hemoglobin that results in
groups, whereas myoglobin only contains one. This heme increased binding affinity for the other three heme groups.
group in both myoglobin and hemoglobin is attached to This cooperative binding mechanism enables hemoglobin
the protein through a distal histidine–iron bond. The Fe(II) to obtain oxygen in the lungs, where there is a high con-
atom protrudes out of the heme plane toward this histi- centration of oxygen, and deliver it to areas of the body
dine. Dioxygen binds to the proximal face through one of with lower concentrations of oxygen. In carbon monox-
◦
its atoms, forming a bent Fe O O bond (>115 ). This ide (CO) poisoning, CO binds to the heme of hemoglobin
bent structure is stabilized by an unligated histidine on the more strongly than dioxygen. In doing so, CO blocks the
proximal heme face, which forms a hydrogen bond to the transport of oxygen to cells. This mechanism of CO toxic-
oxygen atom that is not bound to the iron. When dioxygen ity is less dangerous than the cyanide poisoning described
binds, there is evidence that it is reduced by the iron atom above for cytochrome oxidase.
forming an Fe(III)–superoxide complex. These changes Hemerythrin is an iron dimer containing eight subunits
to the iron cause the metal to move within the heme plane and is about twice as large as hemoglobin. Each iron is