Page 56 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 56
P1: LLL Revised Pages
Encyclopedia of Physical Science and Technology EN002c-73 May 21, 2001 13:59
Boron Hydrides 305
number of three-center bonds equals the number of boron
atoms in the chemical formula, and this result is general
for the entire family of neutral compounds.
It is clear that B 2 H 6 has two boron atoms and two three-
center bonds. The next boron hydride, B 4 H 10 , has four
boron-hydrogen-boron bridges to balance the four boron
atoms. The situation becomes more complex when we
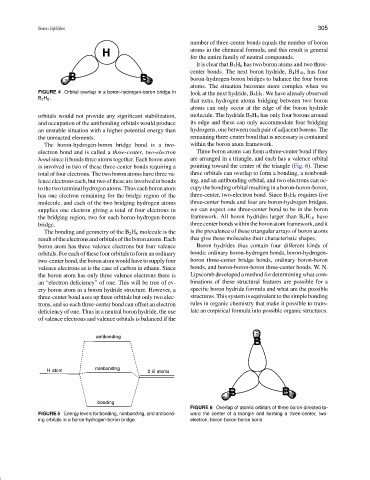
FIGURE 4 Orbital overlap in a boron-hydrogen-boron bridge in look at the next hydride, B 5 H 9 . We have already observed
B 2 H 6 .
that extra hydrogen atoms bridging between two boron
atoms can only occur at the edge of the boron hydride
orbitals would not provide any significant stabilization, molecule. The hydride B 5 H 9 has only four borons around
and occupation of the antibonding orbitals would produce its edge and these can only accommodate four bridging
an unstable situation with a higher potential energy than hydrogens, one between each pair of adjacent borons. The
the unreacted elements. remaining three-center bond that is necessary is contained
The boron-hydrogen-boron bridge bond is a two- within the boron atom framework.
electron bond and is called a three-center, two-electron Three boron atoms can form a three-center bond if they
bond since it bonds three atoms together. Each boron atom are arranged in a triangle, and each has a valence orbital
is involved in two of these three-center bonds requiring a pointing toward the center of the triangle (Fig. 6). These
total of four electrons. The two boron atoms have three va- three orbitals can overlap to form a bonding, a nonbond-
lence electrons each, but two of these are involved in bonds ing, and an antibonding orbital, and two electrons can oc-
to the two terminal hydrogen atoms. Thus each boron atom cupy the bonding orbital resulting in a boron-boron-boron,
has one electron remaining for the bridge region of the three-center, two-electron bond. Since B 5 H 9 requires five
molecule, and each of the two bridging hydrogen atoms three-center bonds and four are boron-hydrogen bridges,
supplies one electron giving a total of four electrons in we can expect one three-center bond to be in the boron
the bridging region, two for each boron-hydrogen-boron framework. All boron hydrides larger than B 4 H 10 have
bridge. threecenterbondswithintheboronatomframework,andit
The bonding and geometry of the B 2 H 6 molecule is the is the prevalence of these triangular arrays of boron atoms
resultoftheelectronsandorbitalsoftheboronatoms.Each that give these molecules their characteristic shapes.
boron atom has three valence electrons but four valence Boron hydrides thus contain four different kinds of
orbitals. For each of these four orbitals to form an ordinary bonds: ordinary boron-hydrogen bonds, boron-hydrogen-
two-centerbond,theboronatomwouldhavetosupplyfour boron three-center bridge bonds, ordinary boron-boron
valence electrons as is the case of carbon in ethane. Since bonds, and boron-boron-boron three-center bonds. W. N.
the boron atom has only three valence electrons there is Lipscomb developed a method for determining what com-
an “electron deficiency” of one. This will be true of ev- binations of these structural features are possible for a
ery boron atom in a boron hydride structure. However, a specific boron hydride formula and what are the possible
three-center bond uses up three orbitals but only two elec- structures. This system is equivalent to the simple bonding
trons, and so each three-center bond can offset an electron rules in organic chemistry that make it possible to trans-
deficiency of one. Thus in a neutral boron hydride, the use late an empirical formula into possible organic structures.
of valence electrons and valence orbitals is balanced if the
FIGURE 6 Overlap of atomic orbitals of three boron directed to-
FIGURE 5 Energy levels for bonding, nonbonding, and antibond- ward the center of a triangle and forming a three-center, two-
ing orbitals in a boron-hydrogen-boron bridge. electron, boron-boron-boron bond.