Page 59 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 59
P1: LLL Revised Pages
Encyclopedia of Physical Science and Technology EN002c-73 May 21, 2001 13:59
308 Boron Hydrides
mentioned previously. See Table II for the number of
electrons needed for each level of structure, closo, nido,
arachno, and hypho. Thus, for example, B 6 H 2− and
6
−
−
B 8 H 2− are closo ions, B 5 H ,B 11 H , and B 10 H − are
8 8 14 13
2−
−
nido ions, B 5 H ,B 5 H , and B 9 H − are arachno ions,
9 10 14
and B 5 H , and B 5 H − are hypho ions.
2−
11 12
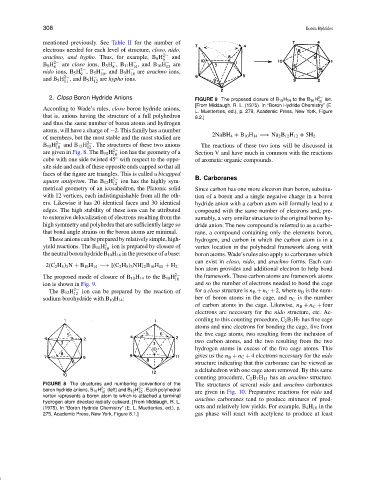
2. Closo Boron Hydride Anions FIGURE 9 The proposed closure of B 10 H 14 to the B 10 H 2− ion.
10
[From Middaugh, R. L. (1975). In “Boron Hydride Chemistry” (E.
According to Wade’s rules, closo boron hydride anions,
L. Muetterties, ed.), p. 279, Academic Press, New York, Figure
that is, anions having the structure of a full polyhedron 8.2.]
and thus the same number of boron atoms and hydrogen
atoms, will have a charge of −2. This family has a number
of members, but the most stable and the most studied are 2NaBH 4 + B 10 H 14 −→ Na 2 B 12 H 12 + 5H 2
B 10 H 2− and B 12 H . The structures of these two anions The reactions of these two ions will be discussed in
2−
10 12
are given in Fig. 8. The B 10 H 2− ion has the geometry of a Section V and have much in common with the reactions
10
cube with one side twisted 45 with respect to the oppo- of aromatic organic compounds.
◦
site side and each of these opposite ends capped so that all
faces of the figure are triangles. This is called a bicapped
square antiprism. The B 12 H 2− ion has the highly sym- B. Carboranes
12
metrical geometry of an icosahedron, the Platonic solid Since carbon has one more electron than boron, substitu-
with 12 vertices, each indistinguishable from all the oth- tion of a boron and a single negative charge in a boron
ers. Likewise it has 20 identical faces and 30 identical hydride anion with a carbon atom will formally lead to a
edges. The high stability of these ions can be attributed compound with the same number of electrons and, pre-
to extensive delocalization of electrons resulting from the sumably, a very similar structure to the original boron hy-
high symmetry and polyhedra that are sufficiently large so dride anion. The new compound is referred to as a carbo-
that bond angle strains on the boron atoms are minimal. rane, a compound containing only the elements boron,
These anions can be prepared by relatively simple, high- hydrogen, and carbon in which the carbon atom is in a
yield reactions. The B 10 H 2− ion is prepared by closure of
10 vertex location in the polyhedral framework along with
the neutral boron hydride B 10 H 14 in the presence of a base: boron atoms. Wade’s rules also apply to carboranes which
can exist in closo, nido, and arachno forms. Each car-
2(C 2 H 5 ) 3 N + B 10 H 14 −→ [(C 2 H 5 ) 3 NH] 2 B 10 H 10 + H 2
bon atom provides and additional electron to help bond
The proposed mode of closure of B 10 H 14 to the B 10 H 2− the framework. These carbon atoms are framework atoms
10
ion is shown in Fig. 9. and so the number of electrons needed to bond the cage
The B 12 H 2− ion can be prepared by the reaction of for a closo structure is n B + n C + 2, where n B is the num-
12
sodium borohydride with B 10 H 14 : ber of boron atoms in the cage, and n C is the number
of carbon atoms in the cage. Likewise, n B + n C + four
electrons are necessary for the nido structure, etc. Ac-
cording to this counting procedure, C 2 B 3 H 7 has five cage
atoms and nine electrons for bonding the cage, five from
the five cage atoms, two resulting from the inclusion of
two carbon atoms, and the two resulting from the two
hydrogen atoms in excess of the five cage atoms. This
gives us the n B + n C + 4 electrons necessary for the nido
structure indicating that this carborane can be viewed as
a deltahedron with one cage atom removed. By this same
counting procedure, C 2 B 7 H 13 has an arachno structure.
FIGURE 8 The structures and numbering conventions of the The structures of several nido and arachno carboranes
boron hydride anions, B 10 H 2− (left) and B 12 H 2− . Each polyhedral
10 12 are given in Fig. 10. Preparative reactions for nido and
vertex represents a boron atom to which is attached a terminal
hydrogen atom directed radially outward. [From Middaugh, R. L. arachno carboranes tend to produce mixtures of prod-
(1975). In “Boron Hydride Chemistry” (E. L. Muetterties, ed.), p. ucts and relatively low yields. For example, B 4 H 10 in the
275, Academic Press, New York, Figure 8.1.] gas phase will react with acetylene to produce at least