Page 54 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 54
P1: LLL Revised Pages
Encyclopedia of Physical Science and Technology EN002c-73 May 21, 2001 13:59
Boron Hydrides 303
However, making the standard assumption that each bond
drawn between two atoms results from the sharing of a pair
of electrons, the number of valence electrons available for
bonding in B 2 H 6 is insufficient. Thus, the seven B H and
B B bonds would require 7 electron pairs or 14 electrons,
but the total number of valence electrons available is only
12, 3 from each boron atom and 1 from each hydrogen
atom. Thus the ethane structure is unreasonable and is
not observed. Because of the apparent shortage of valence
electrons, boron hydrides are often referred to as “electron
deficient” compounds.
The correct structure of B 2 H 6 was determined by in-
frared spectroscopy, and this was the only boron hydride
structure simple enough to be determined by this means.
The actual B 2 H 6 structure is
H H
H
B B
H H H
In this structure, the two boron atoms and the hydrogen
atoms linking them are coplanar and in the plane of the
paper. The other four hydrogen atoms are coplanar with
the boron atoms in a plane perpendicular to the plane of the
paper. The presence of hydrogen atoms linking or bridging
boron atoms is a unique characteristic of boron hydride
structures.
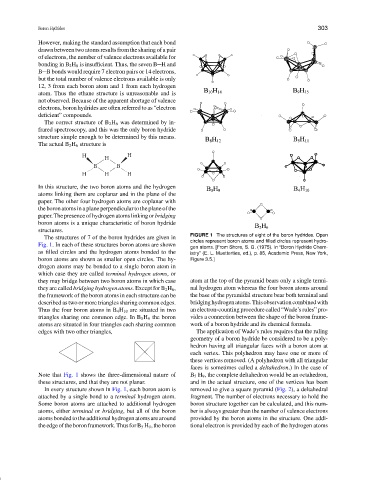
FIGURE 1 The structures of eight of the boron hydrides. Open
The structures of 7 of the boron hydrides are given in
circles represent boron atoms and filled circles represent hydro-
Fig. 1. In each of these structures boron atoms are shown
gen atoms. [From Shore, S. G. (1975). In “Boron Hydride Chem-
as filled circles and the hydrogen atoms bonded to the istry” (E. L. Muetterties, ed.), p. 85, Academic Press, New York,
boron atoms are shown as smaller open circles. The hy- Figure 3.5.]
drogen atoms may be bonded to a single boron atom in
which case they are called terminal hydrogen atoms,or
they may bridge between two boron atoms in which case atom at the top of the pyramid bears only a single termi-
they are called bridging hydrogen atoms. Except for B 2 H 6 , nal hydrogen atom whereas the four boron atoms around
the framework of the boron atoms in each structure can be the base of the pyramidal structure bear both terminal and
described as two or more triangles sharing common edges. bridginghydrogenatoms.Thisobservationcombinedwith
Thus the four boron atoms in B 4 H 10 are situated in two an electron-counting procedure called “Wade’s rules” pro-
triangles sharing one common edge. In B 5 H 9 the boron vides a connection between the shape of the boron frame-
atoms are situated in four triangles each sharing common work of a boron hydride and its chemical formula.
edges with two other triangles, The application of Wade’s rules requires that the ruling
geometry of a boron hydride be considered to be a poly-
hedron having all triangular faces with a boron atom at
each vertex. This polyhedron may have one or more of
these vertices removed. (A polyhedron with all triangular
faces is sometimes called a deltahedron.) In the case of
Note that Fig. 1 shows the three-dimensional nature of B 5 H 9 , the complete deltahedron would be an octahedron,
these structures, and that they are not planar. and in the actual structure, one of the vertices has been
In every structure shown in Fig. 1, each boron atom is removed to give a square pyramid (Fig. 2), a deltahedral
attached by a single bond to a terminal hydrogen atom. fragment. The number of electrons necessary to hold the
Some boron atoms are attached to additional hydrogen boron structure together can be calculated, and this num-
atoms, either terminal or bridging, but all of the boron ber is always greater than the number of valence electrons
atoms bonded to the additional hydrogen atoms are around provided by the boron atoms in the structure. One addi-
theedgeoftheboronframework.ThusforB 5 H 9 ,theboron tional electron is provided by each of the hydrogen atoms