Page 72 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 72
P1: FQP/LPB P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN003D-147 June 13, 2001 22:58
Coordination Compounds 743
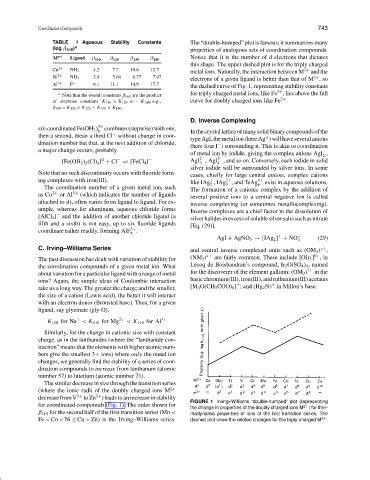
TABLE I Aqueous Stability Constants The “double-humped” plot is famous; it summarizes many
(log β 1n0 ) a properties of analogous sets of coordination compounds.
M n+ + Ligand β 110 β 120 β 130 β 140 Notice that it is the number of d electrons that dictates
this shape. The upper dashed plot is for the triply charged
Cu 2+ NH 3 4.2 7.7 10.6 12.7 metal ions. Naturally, the interaction between M 3+ and the
Ni 2+ NH 3 2.8 5.04 6.77 7.97 electrons of a given ligand is better than that of M ,so
2+
Al 3+ F − 6.1 11.1 14.9 17.7 the dashed curve of Fig. 1, representing stability constants
3+
a Note that the overall constants β 1n0 are the product for triply charged metal ions, like Fe , lies above the full
2+
of stepwise constants K 110 × K 120 ×··· K 1n0 , e.g., curve for doubly charged ions like Fe .
β 140 = K 110 × K 120 × K 130 × K 140 .
D. Inverse Complexing
six-coordinatedFe(OH 2 ) 3+ combines(stepwise)withone,
6 Inthecrystallatticeofmanysolidbinarycompoundsofthe
−
then a second, thean a third Cl without change in coor-
+
type AgI, the metal ion (here Ag ) will have several anions
dination number but that, at the next addition of chloride,
(here four I ) surrounding it. This is akin to coordination
−
a major change occurs, probably
of metal ion by iodide, giving the complex anions AgI ,
−
2
3−
0
2−
−
[Fe(OH 2 ) (Cl) ] + Cl [FeCl 4 ] − AgI , AgI , and so on. Conversely, each iodide in solid
4
3
3
3
silver iodide will be surrounded by silver ions. In some
Note that no such discontinuity occurs with fluoride form-
cases, chiefly for large central anions, complex cations
ing complexes with iron(III). + 2+ 4+
like IAg , IAg , and TeAg 6 exist in aqueous solutions.
2
3
The coordination number of a given metal ion, such
The formation of a cationic complex by the addition of
as Co 2+ or Al 3+ (which indicates the number of ligands
several positive ions to a central negative ion is called
attached to it), often varies from ligand to ligand. For ex-
inverse complexing (or sometimes metallocomplexing).
ample, whereas for aluminum, aqueous chloride forms
Inverse complexes are a chief factor in the dissolution of
−
[AlCl 4 ] and the addition of another chloride ligand (a
silverhalidesinexcessofsolublesilversaltssuchasnitrate
fifth and a sixth) is not easy, up to six fluoride ligands [Eq. (29)].
3−
coordinate rather readily, forming AlF .
6
+
AgI + AgNO 3 → [IAg 2 ] + NO − (29)
3
C. Irving–Williams Series and central inverse complexed units such as (OM 3 ) ,
y+
8+
The past discussion has dealt with variation of stability for (NM 3 ) x+ are fairly common. These include [OIr 3 ] ,in
the coordination compounds of a given metal ion. What Lecoq de Boisbaudran’s compound, Ir 3 O(SO 4 ) 4 , named
about variation for a particular ligand with a range of metal for the discoverer of the element gallium; (OM 3 ) 7+ in the
ions? Again, the simple ideas of Coulombic interaction basic chromium(III), iron(III), and ruthenium(III) acetates
+
+
take us a long way. The greater the charge and the smaller, [M 3 O(CH 3 COO) 6 ] ; and (Hg 2 N) in Millon’s base.
the size of a cation (Lewis acid), the better it will interact
with an electron donor (Br¨onsted base). Thus, for a given
ligand, say glycinate (gly-O),
K 110 for Na < K 110 for Mg 2+ < K 110 for Al 3+
+
Similarly, for the change in cationic size with constant
charge, as in the lanthanides (where the “lanthanide con-
traction” means that the elements with higher atomic num-
bers give the smallest 3+ ions) where only the metal ion
changes, we generally find the stability of a series of coor-
dination compounds to increase from lanthanum (atomic
number 57) to lutetium (atomic number 71).
The similar decrease in size through the transition series
(where the ionic radii of the doubly charged ions M 2+
decrease from V 2+ to Zn ) leads to an increase in stability
2+
FIGURE 1 Irving–Williams “double-humped” plot (representing
for coordinated compounds (Fig. 1). The order shown for
the change in properties of the doubly charged ions M 2+ ) for ther-
β 110 for the second half of the first transition series (Mn < modynamic properties of ions of the first transition series. The
Fe < Co < Ni ≤ Cu > Zn) is the Irving–Williams series. dashed plot show the related changes for the triply charged M 3+ .