Page 74 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 74
P1: FQP/LPB P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN003D-147 June 13, 2001 22:58
Coordination Compounds 745
or at equilibrium,
RT [Fe ]
2+
0
E = 2.303 log (38)
3+
nF [Fe ]
3+
The concentrations [Fe ] and [Fe ] here are those of
2+
the free, uncomplexed metal ions (i.e., those in a solvent
environment). Now, what happens if a coordinating lig-
(35)
and X is added? Both M n+ and M (n+1)+ (e.g., Fe 2+ and
Fe ) are bound, but to differing degrees. This depresses
3+
the concentration of the two free (aquated) ions differen-
tially. Such binding by the ligand is described by stability
constants for the two oidation states, β II and β III , so that
110 110
III
RT [Fe(II)X] β 110
0
E = E − 2.303 log II (39)
nF β [Fe(III)X]
110
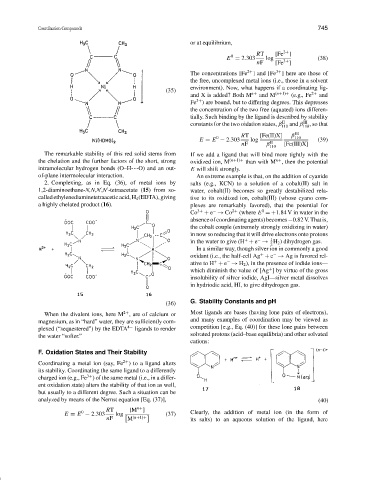
The remarkable stability of this red solid stems from If we add a ligand that will bind more tightly with the
the chelation and the further factors of the short, strong oxidized ion, M (n+1)+ than with M , then the potential
n+
intramolecular hydrogen bonds (O–H---O) and an out- E will shift strongly.
of-plane intermolecular interaction. An extreme example is that, on the addition of cyanide
2. Complexing, as in Eq. (36), of metal ions by salts (e.g., KCN) to a solution of a cobalt(II) salt in
1,2-diaminoethane-N,N,N,N -tetraacetate (15) from so- water, cobalt(II) becomes so greatly destabilized rela-
called ethylenediaminetetraacetic acid, H 4 (EDTA), giving tive to its oxidized ion, cobalt(III) (whose cyano com-
a highly chelated product (16). plexes are remarkably favored), that the potential for
0
Co 3+ + e → Co 2+ (where E =+1.84 V in water in the
−
absence of coordinating agents) becomes −0.82 V. That is,
the cobalt couple (extremely strongly oxidizing in water)
in now so reducing that it will drive electrons onto protons
1
−
+
in the water to give (H + e → H 2 ) dihydrogen gas.
2
In a similar way, though silver ion in commonly a good
oxidant (i.e., the half-cell Ag + e → Ag is favored rel-
−
+
ative to H + e → H 2 ), in the presence of iodide ions—
+
−
which diminish the value of [Ag ] by virtue of the gross
+
insolubility of silver iodide, AgI—silver metal dissolves
in hydriodic acid, HI, to give dihydrogen gas.
G. Stability Constants and pH
(36)
Most ligands are bases (having lone pairs of electrons),
2+
When the divalent ions, here M , are of calcium or
and many examples of coordination may be viewed as
magnesium, as in “hard” water, they are sufficiently com-
competition [e.g., Eq. (40)] for these lone pairs between
plexed (“sequestered”) by the EDTA 4− ligands to render
the water “softer.” solvated protons (acid–base equilibria) and other solvated
cations:
F. Oxidation States and Their Stability
Coordinating a metal ion (say, Fe ) to a ligand alters
2+
its stability. Coordinating the same ligand to a differently
charged ion (e.g., Fe ) of the same metal (i.e., in a differ-
3+
ent oxidation state) alters the stability of that ion as well,
but usually to a different degree. Such a situation can be
analyzed by means of the Nernst equation [Eq. (37)], (40)
RT [M ]
n+
0
E = E − 2.303 log (37) Clearly, the addition of metal ion (in the form of
nF M (n+1)+ its salts) to an aqueous solution of the ligand, here