Page 76 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 76
P1: FQP/LPB P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN003D-147 June 13, 2001 22:58
Coordination Compounds 747
[d yz (Fig. 2a, 19), d xz (20), and d xy (21)] have electron
density as far from
the ligand electron density as possible, and hence are
nonbonding.
2. The d x −y orbital (Fig. 2b) typifies those that point
2
2
directly at the ligands: These two (d x −y ,d z ) can overlap
2
2
2
with ligand orbitals and form good bonds along the axes.
From these two metal-centered d orbitals and the compos-
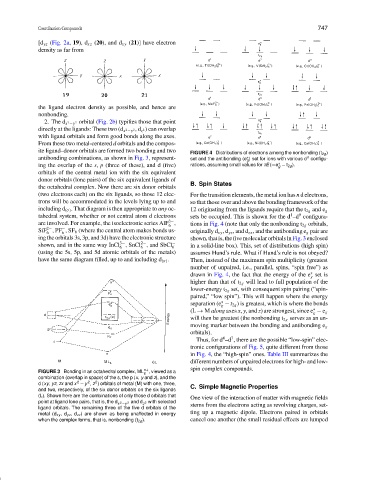
ite ligand–donor orbitals are formed two bonding and two FIGURE 4 Distributions of electrons among the nonbonding (t 2g )
n
antibonding combinations, as shown in Fig. 3, represent- set and the antibonding (e ) set for ions with various d configu-
∗
g
ing the overlap of the s, p (three of these), and d (five) rations, assuming small values for δE (=e − t 2g ).
∗
g
orbitals of the central metal ion with the six equivalent
donor orbitals (lone pairs) of the six equivalent ligands of B. Spin States
the octahedral complex. Now there are six donor orbitals
(two electrons each) on the six ligands, so those 12 elec- For the transition elements, the metal ion has n d electrons,
trons will be accommodated in the levels lying up to and so that those over and above the bonding framework of the
including d (σ) . That diagram is then appropriate to any oc- 12 originating from the ligands require that the t 2g and e g
9
1
tahedral system, whether or not central atom d electrons sets be occupied. This is shown for the d –d configura-
3−
are involved. For example, the isoelectronic series AlF , tions in Fig. 4 (note that only the nonbonding t 2g orbitals,
6
−
SiF ,PF ,SF 6 (where the central atom makes bonds us- originally d xy ,d yz , and d zx , and the antibonding e g pair are
2−
6 6
ing the orbitals 3s, 3p, and 3d) have the electronic structure shown,thatis,thefivemolecularorbitalsinFig.3enclosed
2−
3−
shown, and in the same way InCl , SnCl , and SbCl − in a solid-line box). This, set of distributions (high spin)
6 6 6
(using the 5s, 5p, and 5d atomic orbitals of the metals) assumes Hund’s rule. What if Hund’s rule is not obeyed?
have the same diagram filled, up to and including d (σ) . Then, instead of the maximum spin multiplicity (greatest
number of unpaired, i.e., parallel, spins, “spin free”)as
∗
drawn in Fig. 4, the fact that the energy of the e set is
g
higher than that of t 2g will lead to full population of the
lower-energy t 2g set, with consequent spin pairing (“spin-
paired,”“low spin”). This will happen where the energy
∗
separation (e − t 2g ) is greatest, which is where the bonds
g
∗
(L → M along axes x, y, and z) are strongest, since e − e g
g
will then be greatest (the nonbonding t 2g serves as an un-
moving marker between the bonding and antibonding e g
orbitals).
4
7
Thus, for d –d , there are the possible “low-spin” elec-
tronic configurations of Fig. 5, quite different from those
in Fig. 4, the “high-spin” ones. Table III summarizes the
different numbers of unpaired electrons for high- and low-
spin complex compounds.
FIGURE 3 Bonding in an octahedral complex, ML n+ , viewed as a
6
combination (overlap in space) of the s, the p (x, y and z), and the
2
2
2
d(xy, yz, zx and x − y , z ) orbitals of metal (M) with one, three,
C. Simple Magnetic Properties
and two, respectively, of the six donor orbitals on the six ligands
(L). Shown here are the combinations of only those d orbitals that
One view of the interaction of matter with magnetic fields
point at ligand lone pairs, that is, the d 2 −y 2 and d 2 with selected
x z stems from the electrons acting as revolving charges, set-
ligand orbitals. The remaining three of the five d orbitals of the
ting up a magnetic dipole. Electrons paired in orbitals
metal (d xy ,d yz ,d zx ) are shown as being unaffected in energy
when the complex forms, that is, nonbonding (t 2g ). cancel one another (the small residual effects are lumped