Page 165 - Energy from Toxic Organic Waste for Heat and Power Generation
P. 165
Cracking of Toxic Waste 145
and nitrogen through the use of additional chlorine or sodium hypochlorite,
but at a lower pH level than that used in the first treatment stage.
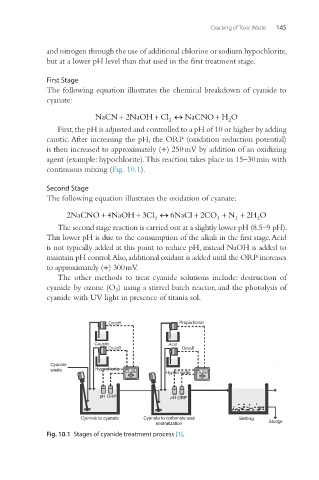
First Stage
The following equation illustrates the chemical breakdown of cyanide to
cyanate:
NaCN + 2NaOH Cl ↔ NaCNOH O
+
+
2
2
First, the pH is adjusted and controlled to a pH of 10 or higher by adding
caustic. After increasing the pH, the ORP (oxidation reduction potential)
is then increased to approximately (+) 250 mV by addition of an oxidizing
agent (example: hypochlorite). This reaction takes place in 15–30 min with
continuous mixing (Fig. 10.1).
Second Stage
The following equation illustrates the oxidation of cyanate:
2NaCNO4NaOH 3Cl ↔ 6NaCl + 2CO + N + 2H O
+
+
2
2
2
2
The second stage reaction is carried out at a slightly lower pH (8.5–9 pH).
This lower pH is due to the consumption of the alkali in the first stage. Acid
is not typically added at this point to reduce pH, instead NaOH is added to
maintain pH control. Also, additional oxidant is added until the ORP increases
to approximately (+) 300 mV.
The other methods to treat cyanide solutions include: destruction of
cyanide by ozone (O 3 ) using a stirred batch reactor, and the photolysis of
cyanide with UV light in presence of titania sol.
On/off Proportional
Caustic Acid
On/off On/off
Cyanide
waste Hypochlorite 5.00 Hypochlorite 5.00
pH ORP pH ORP
Cyanide to cyanate Cyanate to carbonate and Settling
neutralization Sludge
Fig. 10.1 Stages of cyanide treatment process [1].