Page 195 - Engineered Interfaces in Fiber Reinforced Composites
P. 195
Chapter 5. Surface treatments of fibers and effects on composite properties 177
(a) R-SiX3+ HzO - R-Si(OH)j + 3HX
P
HO-5-0 H R R S R
I
I
I
.A 0-Si- 0-S i-0 0-si-0-4-0
H,oH bc1 i i
A A l l M M
P I_.. P
.I_ r,,
Glass
(b) (C) (d)
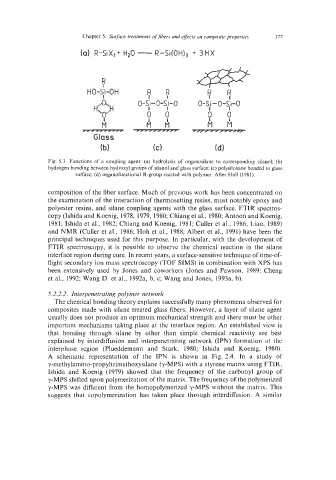
Fig. 5.3. Functions of a coupling agent: (a) hydrolysis of organosilane to corresponding silanol; (b)
hydrogen bonding between hydroxyl groups of silanol and glass surface; (c) polysiloxane bonded to glass
surface; (d) organofunctional R-group reacted with polymer. After Hull (1981).
composition of the fiber surface. Much of previous work has been concentrated on
the examination of the interaction of thermosetting resins, most notably epoxy and
polyester resins, and silane coupling agents with the glass surface. FTIR spectros-
copy (Ishida and Koenig, 1978, 1979, 1980; Chiang et al., 1980; Antoon and Koenig,
1981; Ishida et al., 1982; Chiang and Koenig, 1981; Culler et al., 1986; Liao, 1989)
and NMR (Culler et al., 1986; Hoh et al., 1988; Albert et al., 1991) have been the
principal techniques used for this purpose. In particular, with the development of
FTIR spectroscopy, it is possible to observe the chemical reaction in the silane
interface region during cure. In recent years, a surface-sensitive technique of time-of-
flight secondary ion mass spectroscopy (TOF SIMS) in combination with XPS has
been extensively used by Jones and coworkers (Jones and Pawson, 1989; Cheng
et al., 1992; Wang D. et al., 1992a, b, c; Wang and Jones, 1993a, b).
5.2.2.2. Interpenetrating polymer network
The chemical bonding theory explains successfully many phenomena observed for
composites made with silane treated glass fibers. However, a layer of silane agent
usually does not produce an optimum mechanical strength and there must be other
important mechanisms taking place at the interface region. An established view is
that bonding through silane by other than simple chemical reactivity are best
explained by interdiffusion and interpenetrating network (IPN) formation at the
interphase region (Plueddemann and Stark, 1980; Ishida and Koenig, 1980).
A schematic representation of the IPN is shown in Fig. 2.4. In a study of
y-methylamino-propyltrimethoxysilane (y-MPS) with a styrene matrix using FTIR,
Ishida and Koenig (1979) showed that the frequency of the carbonyl group of
y-MPS shifted upon polymerization of the matrix. The frequency of the polymerized
y-MPS was different from the homopolymerized y-MPS without the matrix. This
suggests that copolymcrization has taken place through interdiffusion. A similar