Page 183 - Engineering Plastics Handbook
P. 183
156 Engineering Plastics
■ High oxygen index and low smoke
■ Medical- and food-contact compliance
■ Sterilizable, and autoclave resistance
■ Good electrical properties with low ion content
■ Excellent machinability and secondary-finishing characteristics
Polymerization Chemistry
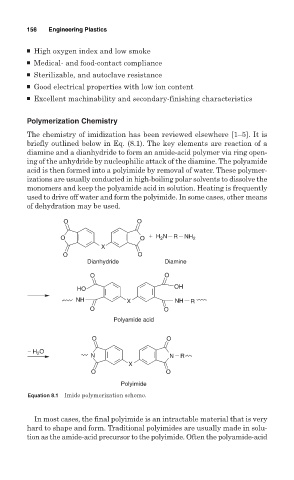
The chemistry of imidization has been reviewed elsewhere [1–5]. It is
briefly outlined below in Eq. (8.1). The key elements are reaction of a
diamine and a dianhydride to form an amide-acid polymer via ring open-
ing of the anhydride by nucleophilic attack of the diamine. The polyamide
acid is then formed into a polyimide by removal of water. These polymer-
izations are usually conducted in high-boiling polar solvents to dissolve the
monomers and keep the polyamide acid in solution. Heating is frequently
used to drive off water and form the polyimide. In some cases, other means
of dehydration may be used.
O O
O O + H 2 N R NH 2
X
O O
Dianhydride Diamine
O O
HO OH
NH X NH R
O O
Polyamide acid
O O
− H 2 O
N N R
X
O O
Polyimide
Equation 8.1 Imide polymerization scheme.
In most cases, the final polyimide is an intractable material that is very
hard to shape and form. Traditional polyimides are usually made in solu-
tion as the amide-acid precursor to the polyimide. Often the polyamide-acid