Page 186 - Engineering Plastics Handbook
P. 186
Thermoplastic Polyetherimide (PEI) 159
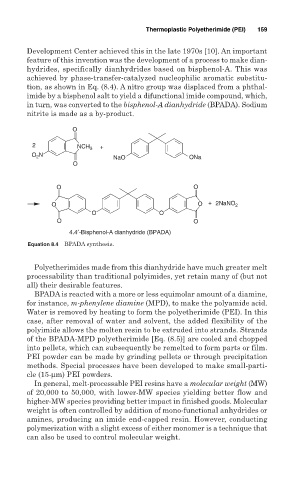
Development Center achieved this in the late 1970s [10]. An important
feature of this invention was the development of a process to make dian-
hydrides, specifically dianhydrides based on bisphenol-A. This was
achieved by phase-transfer-catalyzed nucleophilic aromatic substitu-
tion, as shown in Eq. (8.4). A nitro group was displaced from a phthal-
imide by a bisphenol salt to yield a difunctional imide compound, which,
in turn, was converted to the bisphenol-A dianhydride (BPADA). Sodium
nitrite is made as a by-product.
O
2 NCH 3 +
O N NaO ONa
2
O
O O
O O + 2NaNO 2
O O
O O
4.4′-Bisphenol-A dianhydride (BPADA)
Equation 8.4 BPADA synthesis.
Polyetherimides made from this dianhydride have much greater melt
processability than traditional polyimides, yet retain many of (but not
all) their desirable features.
BPADA is reacted with a more or less equimolar amount of a diamine,
for instance, m-phenylene diamine (MPD), to make the polyamide acid.
Water is removed by heating to form the polyetherimide (PEI). In this
case, after removal of water and solvent, the added flexibility of the
polyimide allows the molten resin to be extruded into strands. Strands
of the BPADA-MPD polyetherimide [Eq. (8.5)] are cooled and chopped
into pellets, which can subsequently be remelted to form parts or film.
PEI powder can be made by grinding pellets or through precipitation
methods. Special processes have been developed to make small-parti-
cle (15-µm) PEI powders.
In general, melt-processable PEI resins have a molecular weight (MW)
of 20,000 to 50,000, with lower-MW species yielding better flow and
higher-MW species providing better impact in finished goods. Molecular
weight is often controlled by addition of mono-functional anhydrides or
amines, producing an imide end-capped resin. However, conducting
polymerization with a slight excess of either monomer is a technique that
can also be used to control molecular weight.