Page 187 - Engineering Plastics Handbook
P. 187
160 Engineering Plastics
It is common practice to combine two polymers with the same over-
all chemical structure, but differing molecular weights, to control the
melt viscosity of the resultant resin. This can be accomplished in sev-
eral different ways, e.g., by blending pellets and melt-mixing in an
extruder or molding machine.
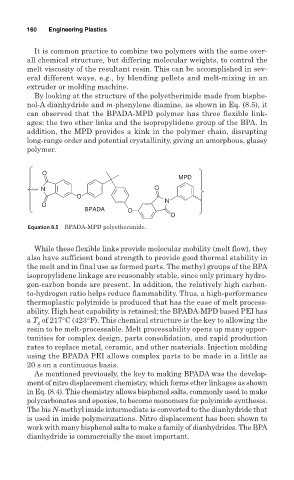
By looking at the structure of the polyetherimide made from bisphe-
nol-A dianhydride and m-phenylene diamine, as shown in Eq. (8.5), it
can observed that the BPADA-MPD polymer has three flexible link-
ages: the two ether links and the isopropylidene group of the BPA. In
addition, the MPD provides a kink in the polymer chain, disrupting
long-range order and potential crystallinity, giving an amorphous, glassy
polymer.
O
MPD
N O
O
N
O
BPADA O
O
Equation 8.5 BPADA-MPD polyetherimide.
While these flexible links provide molecular mobility (melt flow), they
also have sufficient bond strength to provide good thermal stability in
the melt and in final use as formed parts. The methyl groups of the BPA
isopropylidene linkage are reasonably stable, since only primary hydro-
gen-carbon bonds are present. In addition, the relatively high carbon-
to-hydrogen ratio helps reduce flammability. Thus, a high-performance
thermoplastic polyimide is produced that has the ease of melt process-
ability. High heat capability is retained; the BPADA-MPD based PEI has
a T of 217°C (423°F). This chemical structure is the key to allowing the
g
resin to be melt-processable. Melt processability opens up many oppor-
tunities for complex design, parts consolidation, and rapid production
rates to replace metal, ceramic, and other materials. Injection molding
using the BPADA PEI allows complex parts to be made in a little as
20 s on a continuous basis.
As mentioned previously, the key to making BPADA was the develop-
ment of nitro displacement chemistry, which forms ether linkages as shown
in Eq. (8.4). This chemistry allows bisphenol salts, commonly used to make
polycarbonates and epoxies, to become monomers for polyimide synthesis.
The bis N-methyl imide intermediate is converted to the dianhydride that
is used in imide polymerizations. Nitro displacement has been shown to
work with many bisphenol salts to make a family of dianhydrides. The BPA
dianhydride is commercially the most important.