Page 444 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 444
412 Enhanced Oil Recovery in Shale and Tight Reservoirs
13.3.4 Exothermic and endothermic behavior
To define reaction schemes, exothermic and endothermic behavior needs to
be understood; their data can be obtained experimentally by differential
thermal analyzer (DTA), DSC, and accelerating rate calorimetry (ARC).
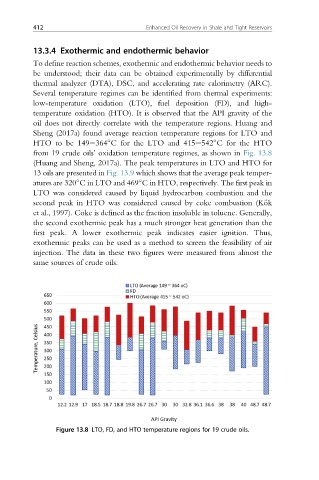
Several temperature regimes can be identified from thermal experiments:
low-temperature oxidation (LTO), fuel deposition (FD), and high-
temperature oxidation (HTO). It is observed that the API gravity of the
oil does not directly correlate with the temperature regions. Huang and
Sheng (2017a) found average reaction temperature regions for LTO and
HTO to be 149e364 C for the LTO and 415e542 C for the HTO
from 19 crude oils’ oxidation temperature regimes, as shown in Fig. 13.8
(Huang and Sheng, 2017a). The peak temperatures in LTO and HTO for
13 oils are presented in Fig. 13.9 which shows that the average peak temper-
atures are 320 C in LTO and 469 C in HTO, respectively. The first peak in
LTO was considered caused by liquid hydrocarbon combustion and the
second peak in HTO was considered caused by coke combustion (K€ok
et al., 1997). Coke is defined as the fraction insoluble in toluene. Generally,
the second exothermic peak has a much stronger heat generation than the
first peak. A lower exothermic peak indicates easier ignition. Thus,
exothermic peaks can be used as a method to screen the feasibility of air
injection. The data in these two figures were measured from almost the
same sources of crude oils.
Figure 13.8 LTO, FD, and HTO temperature regions for 19 crude oils.