Page 445 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 445
Air injection 413
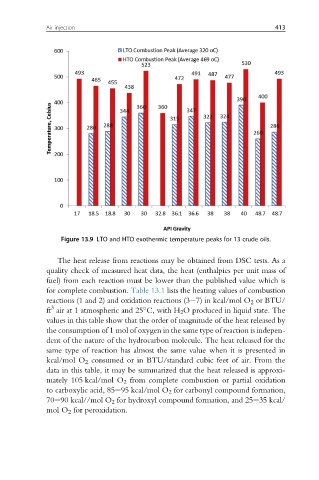
Figure 13.9 LTO and HTO exothermic temperature peaks for 13 crude oils.
The heat release from reactions may be obtained from DSC tests. As a
quality check of measured heat data, the heat (enthalpies per unit mass of
fuel) from each reaction must be lower than the published value which is
for complete combustion. Table 13.1 lists the heating values of combustion
reactions (1 and 2) and oxidation reactions (3e7) in kcal/mol O 2 or BTU/
3
ft air at 1 atmospheric and 25 C, with H 2 O produced in liquid state. The
values in this table show that the order of magnitude of the heat released by
the consumption of 1 mol of oxygen in the same type of reaction is indepen-
dent of the nature of the hydrocarbon molecule. The heat released for the
same type of reaction has almost the same value when it is presented in
kcal/mol O 2 consumed or in BTU/standard cubic feet of air. From the
data in this table, it may be summarized that the heat released is approxi-
mately 105 kcal/mol O 2 from complete combustion or partial oxidation
to carboxylic acid, 85e95 kcal/mol O 2 for carbonyl compound formation,
70e90 kcal//mol O 2 for hydroxyl compound formation, and 25e35 kcal/
mol O 2 for peroxidation.