Page 108 - Entrophy Analysis in Thermal Engineering Systems
P. 108
Irreversible engines—Closed cycles 101
∗
Differentiating Φ with respect to η, we get
dΦ ∗ 1
¼ T R + (7.52)
dη π 1 ηÞ 2
ð
We only need to show that the right-hand side of Eq. (7.52) is always neg-
ative. For this, we use Eq. (5.7), where 1 η¼T 1 /T 2 . Also, because T R <π,
we may write
" #
2
1 1 1 2 T 2
T R + < T R + ¼ T
2 2
ð
π 1 ηÞ T R 1 ηð Þ T R R T 1
1 2 2
3
¼ T T 2 < 0
2
T 1
T R
∗
We therefore conclude that in an ideal Brayton cycle dΦ /dη<0. In other
words, an increase in the thermal efficiency corresponds to a decrease in the
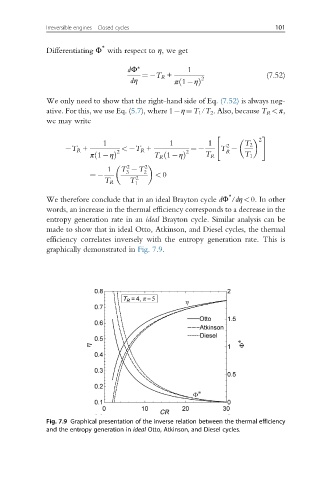
entropy generation rate in an ideal Brayton cycle. Similar analysis can be
made to show that in ideal Otto, Atkinson, and Diesel cycles, the thermal
efficiency correlates inversely with the entropy generation rate. This is
graphically demonstrated in Fig. 7.9.
Fig. 7.9 Graphical presentation of the inverse relation between the thermal efficiency
and the entropy generation in ideal Otto, Atkinson, and Diesel cycles.