Page 70 - Entrophy Analysis in Thermal Engineering Systems
P. 70
62 Entropy Analysis in Thermal Engineering Systems
2!3, adiabatic expansion 3!4, and isochoric heat removal. For this cycle,
we have p 2 ¼p 3 , V 4 ¼V 1 , CR¼V 1 /V 2 , PR¼p 2 /p 1 , and T R ¼T 3 /T 1 . The
thermal efficiency of the cycle can be described as
1 T 4 =T 1 1
q 41
η ¼ 1 ¼ 1 (5.24)
q 23 γ T 3 =T 1 T 2 =T 1
For the adiabatic processes 1!2 and 3!4, we have
1 γ
T 2 V 2 γ 1
¼ ¼ CR (5.25)
T 1 V 1
1 γ 1 γ γ 1 γ
T 4 V 4 V 1 T 2 CR
¼ ¼ ¼ (5.26)
T 3 V 3 V 2 T 3 T R
Note that Eq. (5.25) and the relation V 3 /V 2 ¼T 3 /T 2 are used in Eq. (5.26).
The thermal efficiency of the Diesel cycle can now be obtained in terms of
T R and CR using Eqs. (5.25) and (5.26) in Eq. (5.24). Hence,
γ γ 1 γ
" #
1 T CR Þ 1
ð
η ¼ 1 R (5.27)
γ T R CR γ 1
1
γ
Like the Atkinson cycle, the relation between CR and PR obeys CR ¼ PR .
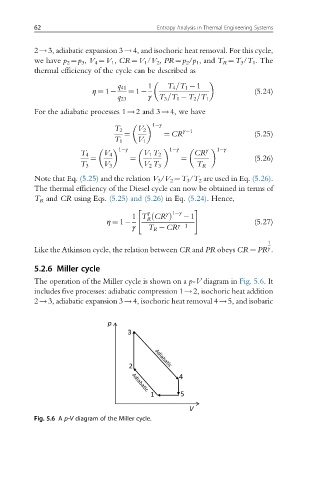
5.2.6 Miller cycle
The operation of the Miller cycle is shown on a p-V diagram in Fig. 5.6.It
includes five processes: adiabatic compression 1!2, isochoric heat addition
2!3, adiabatic expansion 3!4, isochoric heat removal 4!5, and isobaric
Fig. 5.6 A p-V diagram of the Miller cycle.