Page 123 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 123
Methods for Structural and Chemical Characterization of Nanomaterials 109
Anatase
A/B
Rutile
Brookite
Intensity R
B
B A R/B
R A A A
R
A A B B B B R B
R
20 25 30 35 40 45 50 55 60
2 Theta (degree) (Cu Kα source)
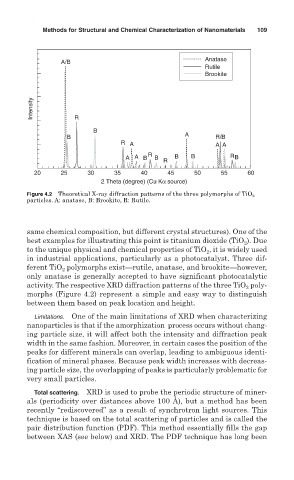
Figure 4.2 Theoretical X-ray diffraction patterns of the three polymorphs of TiO 2
particles. A: anatase, B: Brookite, R: Rutile.
same chemical composition, but different crystal structures). One of the
best examples for illustrating this point is titanium dioxide (TiO ). Due
2
to the unique physical and chemical properties of TiO , it is widely used
2
in industrial applications, particularly as a photocatalyst. Three dif-
ferent TiO polymorphs exist—rutile, anatase, and brookite—however,
2
only anatase is generally accepted to have significant photocatalytic
activity. The respective XRD diffraction patterns of the three TiO poly-
2
morphs (Figure 4.2) represent a simple and easy way to distinguish
between them based on peak location and height.
Limitations. One of the main limitations of XRD when characterizing
nanoparticles is that if the amorphization process occurs without chang-
ing particle size, it will affect both the intensity and diffraction peak
width in the same fashion. Moreover, in certain cases the position of the
peaks for different minerals can overlap, leading to ambiguous identi-
fication of mineral phases. Because peak width increases with decreas-
ing particle size, the overlapping of peaks is particularly problematic for
very small particles.
Total scattering. XRD is used to probe the periodic structure of miner-
als (periodicity over distances above 100 Å), but a method has been
recently “rediscovered” as a result of synchrotron light sources. This
technique is based on the total scattering of particles and is called the
pair distribution function (PDF). This method essentially fills the gap
between XAS (see below) and XRD. The PDF technique has long been