Page 129 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 129
Methods for Structural and Chemical Characterization of Nanomaterials 115
particle the Cd is tetrahedraly coordinated and CdS octahedra appeared
at the surface. XAS can also probe the surface layer structure of particles,
but only if objects are within the nanometer size range. Indeed, XAS and
EXAFS give average information concerning all crystallographic sites
that exist in a mineral. Unfortunately, XAS is only sensitive to the largest
fraction of sites when there are several sites present. In the case of large
particles the surface sites represent a limited number of atoms that
cannot be detected by XAS. As soon as the fraction of surface atoms
becomes higher than 15–20 percent, XAS is sensitive enough to determine
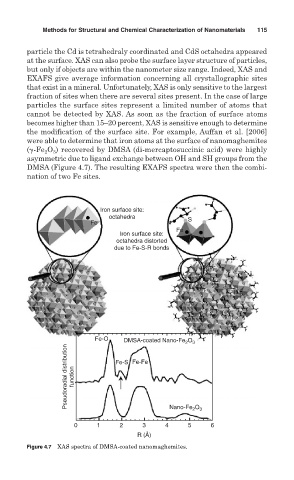
the modification of the surface site. For example, Auffan et al. [2006]
were able to determine that iron atoms at the surface of nanomaghemites
( -Fe 2 O 3 ) recovered by DMSA (di-mercaptosuccinic acid) were highly
asymmetric due to ligand exchange between OH and SH groups from the
DMSA (Figure 4.7). The resulting EXAFS spectra were then the combi-
nation of two Fe sites.
Iron surface site:
octahedra S
Fe
Fe
Iron surface site:
octahedra distorted
due to Fe-S-R bonds
Fe-O DMSA-coated Nano-Fe O 3
2
Pseudoradial distribution function Fe-S Fe-Fe
0 1 2 3 4 Nano-Fe 2 O 3 6
5
R (Å)
Figure 4.7 XAS spectra of DMSA-coated nanomaghemites.