Page 133 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 133
Methods for Structural and Chemical Characterization of Nanomaterials 119
147, 309 atoms (2, 3, or 4 atom shells) with cuboctahedral (fcc.) arrange-
ment and for a given n-shell compound, the “surface” metal atoms in the
th
outer n -shell of the metal core do not show purely “metallic” behavior.
Furthermore, experiments with two shell Au-55 nanoparticles recovered
by different types of ligands (triphenylphosphine, tri-para-tolylphos-
phine, metasulfonatophenyldiphenylphosphine, and trianisylphosphine)
demonstrated that they do not exhibit a perfect metallic character due
to the influence of the surface layer. The charge transfer between the
surface atoms and the ligand influences all the particles even at the core
of the Au-55 particles. For Pt-308 nanoparticles charge transfer between
the ligand and the surface layer did not influence the particle core. In
summary, Mössbauer spectroscopy provides a sensitive local probe to
measure these charge densities and therefore assess the influence of lig-
ands on the surface layer structure.
Nuclear magnetic resonance (NMR)
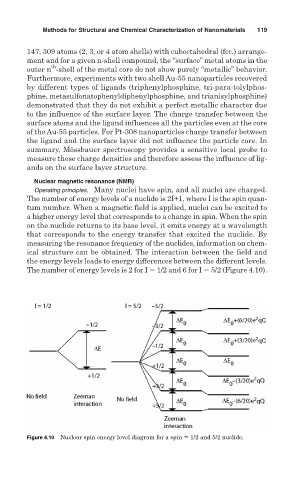
Operating principles. Many nuclei have spin, and all nuclei are charged.
The number of energy levels of a nuclide is 2I+1, where I is the spin quan-
tum number. When a magnetic field is applied, nuclei can be excited to
a higher energy level that corresponds to a change in spin. When the spin
on the nuclide returns to its base level, it emits energy at a wavelength
that corresponds to the energy transfer that excited the nuclide. By
measuring the resonance frequency of the nuclides, information on chem-
ical structure can be obtained. The interaction between the field and
the energy levels leads to energy differences between the different levels.
The number of energy levels is 2 for I 1/2 and 6 for I 5/2 (Figure 4.10).
Figure 4.10 Nuclear spin energy level diagram for a spin 1/2 and 5/2 nuclide.