Page 291 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 291
276 Principles and Methods
120
No EPS
100 Dextran
MWAP71
80
Gellan
Intensity 60
40
20
0
100 1000 10000
a P
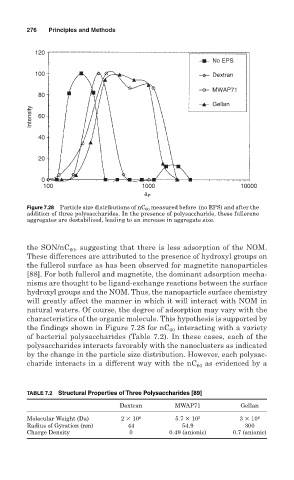
Figure 7.28 Particle size distributions of nC 60 measured before (no EPS) and after the
addition of three polysaccharides. In the presence of polysaccharide, these fullerene
aggregates are destabilized, leading to an increase in aggregate size.
the SON/nC 60 , suggesting that there is less adsorption of the NOM.
These differences are attributed to the presence of hydroxyl groups on
the fullerol surface as has been observed for magnetite nanoparticles
[88]. For both fullerol and magnetite, the dominant adsorption mecha-
nisms are thought to be ligand-exchange reactions between the surface
hydroxyl groups and the NOM. Thus, the nanoparticle surface chemistry
will greatly affect the manner in which it will interact with NOM in
natural waters. Of course, the degree of adsorption may vary with the
characteristics of the organic molecule. This hypothesis is supported by
the findings shown in Figure 7.28 for nC 60 interacting with a variety
of bacterial polysaccharides (Table 7.2). In these cases, each of the
polysaccharides interacts favorably with the nanoclusters as indicated
by the change in the particle size distribution. However, each polysac-
as evidenced by a
charide interacts in a different way with the nC 60
TABLE 7.2 Structural Properties of Three Polysaccharides [89]
Dextran MWAP71 Gellan
Molecular Weight (Da) 2
10 6 5.7
10 5 3
10 6
Radius of Gyration (nm) 44 54.9 300
Charge Density 0 0.49 (anionic) 0.7 (anionic)