Page 294 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 294
Nanoparticle Transport, Aggregation, and Deposition 279
1400
Tannic acid
1200
Blank
1000
800
d (nm)
600
400
200
0
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35
I (M NaCl)
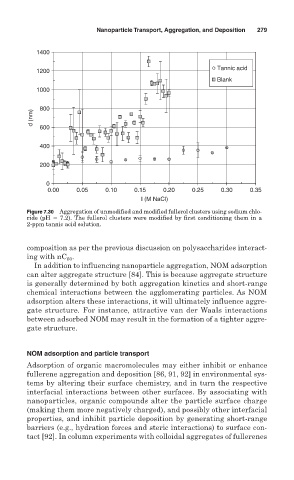
Figure 7.30 Aggregation of unmodified and modified fullerol clusters using sodium chlo-
ride (pH 7.2). The fullerol clusters were modified by first conditioning them in a
2-ppm tannic acid solution.
composition as per the previous discussion on polysaccharides interact-
ing with nC .
60
In addition to influencing nanoparticle aggregation, NOM adsorption
can alter aggregate structure [84]. This is because aggregate structure
is generally determined by both aggregation kinetics and short-range
chemical interactions between the agglomerating particles. As NOM
adsorption alters these interactions, it will ultimately influence aggre-
gate structure. For instance, attractive van der Waals interactions
between adsorbed NOM may result in the formation of a tighter aggre-
gate structure.
NOM adsorption and particle transport
Adsorption of organic macromolecules may either inhibit or enhance
fullerene aggregation and deposition [86, 91, 92] in environmental sys-
tems by altering their surface chemistry, and in turn the respective
interfacial interactions between other surfaces. By associating with
nanoparticles, organic compounds alter the particle surface charge
(making them more negatively charged), and possibly other interfacial
properties, and inhibit particle deposition by generating short-range
barriers (e.g., hydration forces and steric interactions) to surface con-
tact [92]. In column experiments with colloidal aggregates of fullerenes