Page 292 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 292
Nanoparticle Transport, Aggregation, and Deposition 277
different evolution in the size distribution produced in each instance
(Figure 7.28).
The unique aggregation results found observed for the various poly-
saccharides in Figure 7.28 may be traced to their different molecular
chemistries and do not demonstrate a clear size effect. In this example,
the acetate groups (CH COO ) that are characteristic of the MWAP71
3
and gellan seem to promote aggregation. Conversely, no trend was evi-
denced with regards to molecular size and the degree of aggregation.
Indeed, the smaller MWAP71 produced a similar degree of aggregation
as the larger gellan, while less severe aggregation was seen with the dex-
tran. While not considered as conclusive evidence, these results suggest
that chemical interactions and not purely size must be considered when
evaluating nanoparticle interactions with complex molecules like poly-
sacharrides. In other words, we see that chemistry, and not size alone,
continues to be important.
Beyond merely altering particle surface charge, adsorption of NOM
may affect the type of interactions that occur between particles in water.
For example, the thickness of the adsorbed organic layer may, under spe-
cific conditions (high salinity), exceed the thickness of the diffuse double
layer, ultimately altering the nature of the interfacial interactions
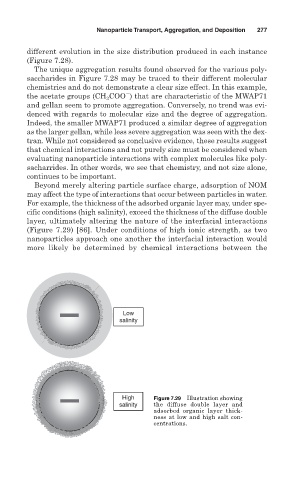
(Figure 7.29) [86]. Under conditions of high ionic strength, as two
nanoparticles approach one another the interfacial interaction would
more likely be determined by chemical interactions between the
Low
salinity
High Figure 7.29 Illustration showing
salinity the diffuse double layer and
adsorbed organic layer thick-
ness at low and high salt con-
centrations.