Page 290 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 290
Nanoparticle Transport, Aggregation, and Deposition 275
of 4 to 6) and ionic strengths of 1 to 10 mM NaCl. Similar conditions may
favor association with other nanoscale particles.
Naturally occurring organic matter
and particle charge
Although the majority of surfaces in aqueous environments carry a net
negative charge, the magnitude of this charge can vary considerably
with differences in functionality and charge density. Adsorption of NOM
to a surface will alter the charge properties of that surface, in most
cases making it more negatively charged [86]. Modification of the par-
ticle surface charge will depend on a variety of factors, including the
charge characteristics of the NOM (e.g., charge density and functional-
ity), the mode of adsorption (inner versus outer sphere) [87], particle sur-
face chemistry, and the solution chemistry.
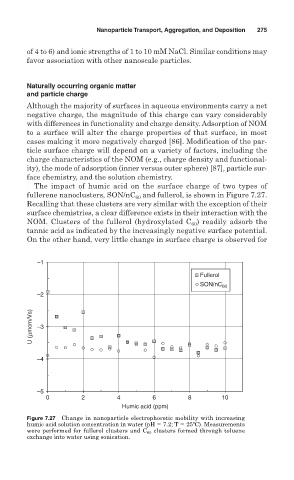
The impact of humic acid on the surface charge of two types of
and fullerol, is shown in Figure 7.27.
fullerene nanoclusters, SON/nC 60
Recalling that these clusters are very similar with the exception of their
surface chemistries, a clear difference exists in their interaction with the
NOM. Clusters of the fullerol (hydroxylated C ) readily adsorb the
60
tannic acid as indicated by the increasingly negative surface potential.
On the other hand, very little change in surface charge is observed for
–1
Fullerol
SON/nC 60
–2
U (mmcm/Vs) –3
–4
–5
0 2 4 6 8 10
Humic acid (ppm)
Figure 7.27 Change in nanoparticle electrophoretic mobility with increasing
humic acid solution concentration in water (pH 7.2; T 25ºC). Measurements
were performed for fullerol clusters and C 60 clusters formed through toluene
exchange into water using sonication.