Page 285 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 285
270 Principles and Methods
1.E–05 1.4
1.2
1.E–06
1.0
1.E–07
U KE /kT 1.E–08 0.8 φ min /kT
0.6
1.E–09
0.4
v = 1 m/day
1.E–10
v = 0.5 m/day 0.2
2nd Minima
1.E–11 0.0
0 100 200 300 400 500
dp (nm)
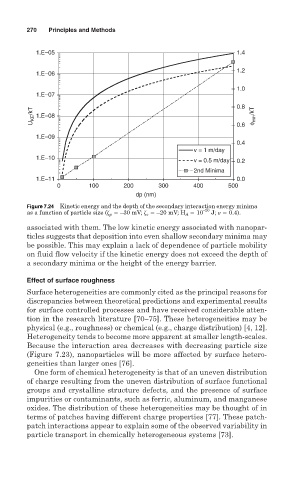
Figure 7.24 Kinetic energy and the depth of the secondary interaction energy minima
as a function of particle size ( p –30 mV; c –20 mV; H A 10 –20 J; 0.4).
associated with them. The low kinetic energy associated with nanopar-
ticles suggests that deposition into even shallow secondary minima may
be possible. This may explain a lack of dependence of particle mobility
on fluid flow velocity if the kinetic energy does not exceed the depth of
a secondary minima or the height of the energy barrier.
Effect of surface roughness
Surface heterogeneities are commonly cited as the principal reasons for
discrepancies between theoretical predictions and experimental results
for surface controlled processes and have received considerable atten-
tion in the research literature [70–75]. These heterogeneities may be
physical (e.g., roughness) or chemical (e.g., charge distribution) [4, 12].
Heterogeneity tends to become more apparent at smaller length-scales.
Because the interaction area decreases with decreasing particle size
(Figure 7.23), nanoparticles will be more affected by surface hetero-
geneities than larger ones [76].
One form of chemical heterogeneity is that of an uneven distribution
of charge resulting from the uneven distribution of surface functional
groups and crystalline structure defects, and the presence of surface
impurities or contaminants, such as ferric, aluminum, and manganese
oxides. The distribution of these heterogeneities may be thought of in
terms of patches having different charge properties [77]. These patch-
patch interactions appear to explain some of the observed variability in
particle transport in chemically heterogeneous systems [73].