Page 315 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 315
300 Environmental Applications of Nanomaterials
or straining and pore plugging will limit nanoparticle transport. While
maximizing transportability, ideally the nanomaterials will have an
affinity for the target contaminant. For example, nanomaterials with
hydrophobic character may partition to the DNAPL/water interface
thereby delivering the particles to where they are most needed (labeled
D in Figure 8.1). A fundamental understanding of the particle properties
controlling the reactivity (rates and products/intermediates) and lifetime
of iron nanoparticles, optimal methods of nanoiron injection/transport
to the contaminant source zone, and methods for targeting specific sub-
surface contaminants or source areas to increase their effectiveness are
needed.
Reactivity, Fate, and Lifetime
Nanoparticles can sequester groundwater contaminants (via adsorption
or complexation), making them immobile, or can degrade or transform
them to innocuous compounds. Contaminant transformations by nano-
iron, which is a strong reductant, are typically redox reactions. When the
oxidant or reductant is the nanoparticle itself, it is considered a reactive
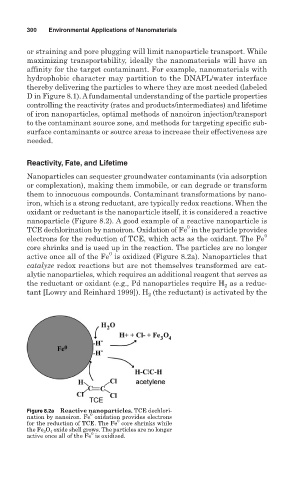
nanoparticle (Figure 8.2). A good example of a reactive nanoparticle is
0
TCE dechlorination by nanoiron. Oxidation of Fe in the particle provides
0
electrons for the reduction of TCE, which acts as the oxidant. The Fe
core shrinks and is used up in the reaction. The particles are no longer
0
active once all of the Fe is oxidized (Figure 8.2a). Nanoparticles that
catalyze redox reactions but are not themselves transformed are cat-
alytic nanoparticles, which requires an additional reagent that serves as
the reductant or oxidant (e.g., Pd nanoparticles require H as a reduc-
2
tant [Lowry and Reinhard 1999]). H (the reductant) is activated by the
2
Figure 8.2a Reactive nanoparticles. TCE dechlori-
0
nation by nanoiron. Fe oxidation provides electrons
0
for the reduction of TCE. The Fe core shrinks while
the Fe 3 O 4 oxide shell grows. The particles are no longer
0
active once all of the Fe is oxidized.