Page 320 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 320
Nanomaterials for Groundwater Remediation 305
insufficient, addition of Pd or some other noble metal catalyst increases
the reactivity, but this also increases the cost, which currently ranges
from $50–110/kg for nanoiron without added catalyst.
Reaction products, intermediates,
and efficiency
There are two reasons why in situ remediation using reactive nanopar-
ticles must consider the reaction products formed. First, a reaction prod-
uct may potentially be more toxic or mobile than the parent compound,
and thus increases the risks posed by the site rather than decreasing
them. Second, the products formed can strongly influence the effec-
tiveness and costs of remediation. Developers of reactive nanoparticles
for remediation need to consider the production and potential negative
consequences of reaction intermediates and products. TCE offers a good
example of how in situ remediation may increase the risks at a site
rather than decrease them. Under reducing conditions afforded by nano-
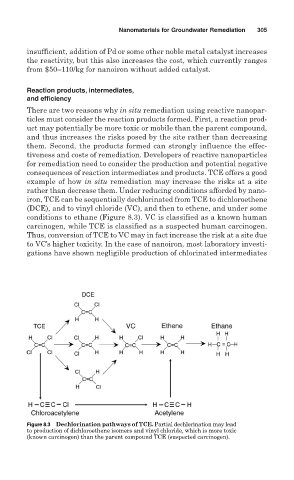
iron, TCE can be sequentially dechlorinated from TCE to dichloroethene
(DCE), and to vinyl chloride (VC), and then to ethene, and under some
conditions to ethane (Figure 8.3). VC is classified as a known human
carcinogen, while TCE is classified as a suspected human carcinogen.
Thus, conversion of TCE to VC may in fact increase the risk at a site due
to VC’s higher toxicity. In the case of nanoiron, most laboratory investi-
gations have shown negligible production of chlorinated intermediates
Figure 8.3 Dechlorination pathways of TCE. Partial dechlorination may lead
to production of dichloroethene isomers and vinyl chloride, which is more toxic
(known carcinogen) than the parent compound TCE (suspected carcinogen).