Page 316 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 316
Nanomaterials for Groundwater Remediation 301
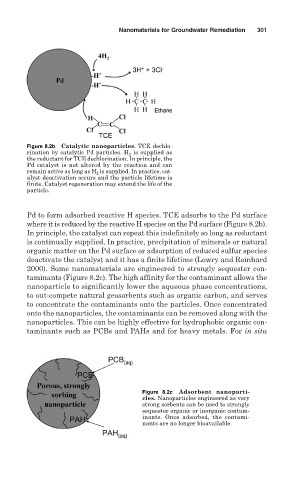
Figure 8.2b Catalytic nanoparticles. TCE dechlo-
rination by catalytic Pd particles. H 2 is supplied as
the reductant for TCE dechlorination. In principle, the
Pd catalyst is not altered by the reaction and can
remain active as long as H 2 is supplied. In practice, cat-
alyst deactivation occurs and the particle lifetime is
finite. Catalyst regeneration may extend the life of the
particle.
Pd to form adsorbed reactive H species. TCE adsorbs to the Pd surface
where it is reduced by the reactive H species on the Pd surface (Figure 8.2b).
In principle, the catalyst can repeat this indefinitely so long as reductant
is continually supplied. In practice, precipitation of minerals or natural
organic matter on the Pd surface or adsorption of reduced sulfur species
deactivate the catalyst and it has a finite lifetime (Lowry and Reinhard
2000). Some nanomaterials are engineered to strongly sequester con-
taminants (Figure 8.2c). The high affinity for the contaminant allows the
nanoparticle to significantly lower the aqueous phase concentrations,
to out-compete natural geosorbents such as organic carbon, and serves
to concentrate the contaminants onto the particles. Once concentrated
onto the nanoparticles, the contaminants can be removed along with the
nanoparticles. This can be highly effective for hydrophobic organic con-
taminants such as PCBs and PAHs and for heavy metals. For in situ
Figure 8.2c Adsorbent nanoparti-
cles. Nanoparticles engineered as very
strong sorbents can be used to strongly
sequester organic or inorganic contam-
inants. Once adsorbed, the contami-
nants are no longer bioavailable.