Page 325 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 325
310 Environmental Applications of Nanomaterials
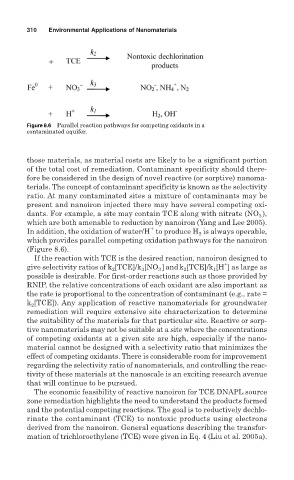
Figure 8.6 Parallel reaction pathways for competing oxidants in a
contaminated aquifer.
those materials, as material costs are likely to be a significant portion
of the total cost of remediation. Contaminant specificity should there-
fore be considered in the design of novel reactive (or sorptive) nanoma-
terials. The concept of contaminant specificity is known as the selectivity
ratio. At many contaminated sites a mixture of contaminants may be
present and nanoiron injected there may have several competing oxi-
dants. For example, a site may contain TCE along with nitrate (NO ),
3
which are both amenable to reduction by nanoiron (Yang and Lee 2005).
In addition, the oxidation of water/H to produce H is always operable,
2
which provides parallel competing oxidation pathways for the nanoiron
(Figure 8.6).
If the reaction with TCE is the desired reaction, nanoiron designed to
+
give selectivity ratios of k [TCE]/k [NO ]andk [TCE]/k [H ] as large as
1
2
3
2
3
possible is desirable. For first-order reactions such as those provided by
RNIP, the relative concentrations of each oxidant are also important as
the rate is proportional to the concentration of contaminant (e.g., rate =
k [TCE]). Any application of reactive nanomaterials for groundwater
2
remediation will require extensive site characterization to determine
the suitability of the materials for that particular site. Reactive or sorp-
tive nanomaterials may not be suitable at a site where the concentrations
of competing oxidants at a given site are high, especially if the nano-
material cannot be designed with a selectivity ratio that minimizes the
effect of competing oxidants. There is considerable room for improvement
regarding the selectivity ratio of nanomaterials, and controlling the reac-
tivity of these materials at the nanoscale is an exciting research avenue
that will continue to be pursued.
The economic feasibility of reactive nanoiron for TCE DNAPL source
zone remediation highlights the need to understand the products formed
and the potential competing reactions. The goal is to reductively dechlo-
rinate the contaminant (TCE) to nontoxic products using electrons
derived from the nanoiron. General equations describing the transfor-
mation of trichloroethylene (TCE) were given in Eq. 4 (Liu et al. 2005a).