Page 322 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 322
Nanomaterials for Groundwater Remediation 307
0
0
RNIP (FeOOH → Fe → Fe /Fe O )
3
4
+ TCE/H 2 O
50 nm
50 nm
0
Fe(B) (Fe 2+ + BH – →Fe /FeB /Na B O )
4
7
2 4
x
+ TCE/H O
2
50 nm
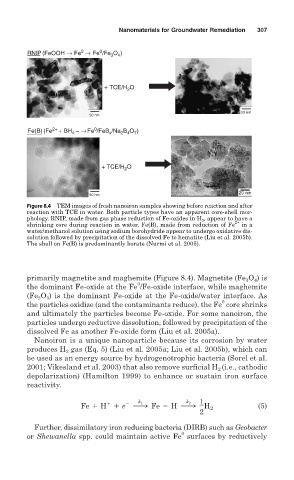
Figure 8.4 TEM images of fresh nanoiron samples showing before reaction and after
reaction with TCE in water. Both particle types have an apparent core-shell mor-
phology. RNIP, made from gas phase reduction of Fe-oxides in H 2 , appear to have a
2+
shrinking core during reaction in water. Fe(B), made from reduction of Fe in a
water/methanol solution using sodium borohydride appear to undergo oxidative dis-
solution followed by precipitation of the dissolved Fe to hematite (Liu et al. 2005b).
The shell on Fe(B) is predominantly borate (Nurmi et al. 2005).
primarily magnetite and maghemite (Figure 8.4). Magnetite (Fe O ) is
4
3
0
the dominant Fe-oxide at the Fe /Fe-oxide interface, while maghemite
(Fe O ) is the dominant Fe-oxide at the Fe-oxide/water interface. As
3
2
0
the particles oxidize (and the contaminants reduce), the Fe core shrinks
and ultimately the particles become Fe-oxide. For some nanoiron, the
particles undergo reductive dissolution, followed by precipitation of the
dissolved Fe as another Fe-oxide form (Liu et al. 2005a).
Nanoiron is a unique nanoparticle because its corrosion by water
produces H gas (Eq. 5) (Liu et al. 2005a; Liu et al. 2005b), which can
2
be used as an energy source by hydrogenotrophic bacteria (Sorel et al.
2001; Vikesland et al. 2003) that also remove surficial H (i.e., cathodic
2
depolarization) (Hamilton 1999) to enhance or sustain iron surface
reactivity.
# k 2 1
2
1
k 1
Fe 1 H 1 e h Fe 2 H h H 2 (5)
2
Further, dissimilatory iron reducing bacteria (DIRB) such as Geobacter
0
or Shewanella spp. could maintain active Fe surfaces by reductively