Page 324 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 324
Nanomaterials for Groundwater Remediation 309
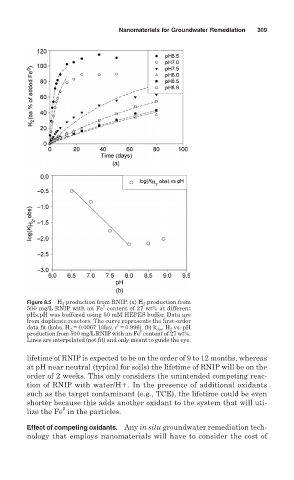
Figure 8.5 H 2 production from RNIP. (a) H 2 production from
0
500 mg/L RNIP with an Fe content of 27 wt% at different
pHs.pH was buffered using 50 mM HEPES buffer. Data are
from duplicate reactors. The curve represents the first-order
2
data fit (kobs, H 2 = 0.0067 1/day, r = 0.996). (b) k obs , H 2 vs. pH
0
production from 500 mg/L RNIP with an Fe content of 27 wt%.
Lines are interpolated (not fit) and only meant to guide the eye.
lifetime of RNIP is expected to be on the order of 9 to 12 months, whereas
at pH near neutral (typical for soils) the lifetime of RNIP will be on the
order of 2 weeks. This only considers the unintended competing reac-
tion of RNIP with water/H . In the presence of additional oxidants
such as the target contaminant (e.g., TCE), the lifetime could be even
shorter because this adds another oxidant to the system that will uti-
0
lize the Fe in the particles.
Effect of competing oxidants. Any in situ groundwater remediation tech-
nology that employs nanomaterials will have to consider the cost of