Page 106 - Essentials of physical chemistry
P. 106
68 Essentials of Physical Chemistry
Precision
0°C–30°C jacket
thermometer
thermometer
(graduated to
(optional)
0.01°C or 0.02°C)
Belt
Stirrer
Ignition
lead
Motor
2 L water
Bomb
(see Figure 4.2)
Water
flow for
jacket
(optional)
Pail
Lead
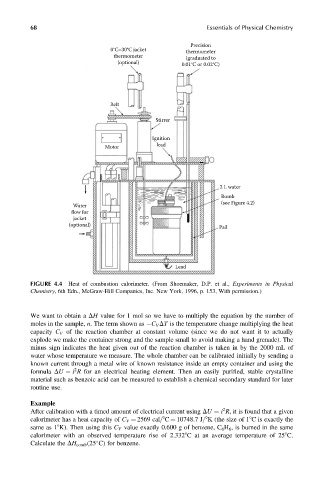
FIGURE 4.4 Heat of combustion calorimeter. (From Shoemaker, D.P. et al., Experiments in Physical
Chemistry, 6th Edn., McGraw-Hill Companies, Inc. New York, 1996, p. 153, With permission.)
We want to obtain a DH value for 1 mol so we have to multiply the equation by the number of
moles in the sample, n. The term shown as C V DT is the temperature change multiplying the heat
capacity C V of the reaction chamber at constant volume (since we do not want it to actually
explode we make the container strong and the sample small to avoid making a hand grenade). The
minus sign indicates the heat given out of the reaction chamber is taken in by the 2000 mL of
water whose temperature we measure. The whole chamber can be calibrated initially by sending a
known current through a metal wire of known resistance inside an empty container and using the
2
formula DU ¼ i R for an electrical heating element. Then an easily purified, stable crystalline
material such as benzoic acid can be measured to establish a chemical secondary standard for later
routine use.
Example
2
After calibration with a timed amount of electrical current using DU ¼ i R, it is found that a given
calorimeter has a heat capacity of C V ¼ 2569 cal=8C ¼ 10748.7 J=8K (the size of 18C is exactly the
same as 18K). Then using this C V value exactly 0.600 g of benzene, C 6 H 6 , is burned in the same
calorimeter with an observed temperature rise of 2.3328C at an average temperature of 258C.
Calculate the DH comb (25 C) for benzene.