Page 110 - Essentials of physical chemistry
P. 110
72 Essentials of Physical Chemistry
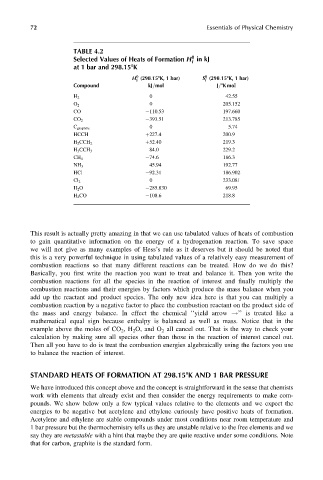
TABLE 4.2
0
Selected Values of Heats of Formation H in kJ
f
at 1 bar and 298.158K
0
0
H (298:158K, 1 bar) S (298:158K, 1 bar)
f f
Compound kJ=mol J=8K mol
0 42.55
H 2
O 2 0 205.152
CO 110.53 197.660
CO 2 393.51 213.785
0 5.74
C graphite
HCCH þ227.4 200.9
þ52.40 219.3
H 2 CCH 2
84.0 229.2
H 3 CCH 3
74.6 186.3
CH 4
45.94 192.77
NH 3
HCl 92.31 186.902
0 233.081
Cl 2
H 2 O 285.830 69.95
H 2 CO 108.6 218.8
This result is actually pretty amazing in that we can use tabulated values of heats of combustion
to gain quantitative information on the energy of a hydrogenation reaction. To save space
we will not give as many examples of Hess’s rule as it deserves but it should be noted that
this is a very powerful technique in using tabulated values of a relatively easy measurement of
combustion reactions so that many different reactions can be treated. How do we do this?
Basically, you first write the reaction you want to treat and balance it. Then you write the
combustion reactions for all the species in the reaction of interest and finally multiply the
combustion reactions and their energies by factors which produce the mass balance when you
add up the reactant and product species. The only new idea here is that you can multiply a
combustion reaction by a negative factor to place the combustion reactant on the product side of
the mass and energy balance. In effect the chemical ‘‘yield arrow !’’ is treated like a
mathematical equal sign because enthalpy is balanced as well as mass. Notice that in the
example above the moles of CO 2 ,H 2 O, and O 2 all cancel out. That is the way to check your
calculation by making sure all species other than those in the reaction of interest cancel out.
Then all you have to do is treat the combustion energies algebraically using the factors you use
to balance the reaction of interest.
STANDARD HEATS OF FORMATION AT 298.158K AND 1 BAR PRESSURE
We have introduced this concept above and the concept is straightforward in the sense that chemists
work with elements that already exist and then consider the energy requirements to make com-
pounds. We show below only a few typical values relative to the elements and we expect the
energies to be negative but acetylene and ethylene curiously have positive heats of formation.
Acetylene and ethylene are stable compounds under most conditions near room temperature and
1 bar pressure but the thermochemistry tells us they are unstable relative to the free elements and we
say they are metastable with a hint that maybe they are quite reactive under some conditions. Note
that for carbon, graphite is the standard form.