Page 158 - Essentials of physical chemistry
P. 158
120 Essentials of Physical Chemistry
1150
1001.6
Volume, mL 1100 1001.4
1050 Volume, mL 1001.2
1000
1001.0
0 2 4 6 8 0 0.05 0.1 0.15 0.2 0.25
per 1000 g H O Moles MgSO per 1000 g H O
(a) Moles NH 3 (b)
2 4 2
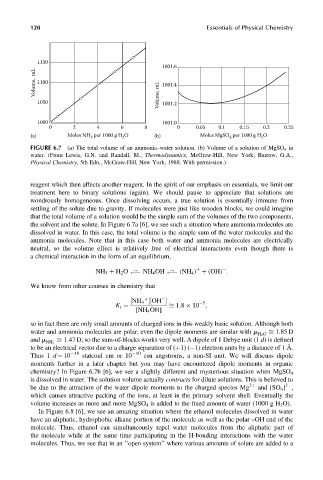
FIGURE 6.7 (a) The total volume of an ammonia–water solution. (b) Volume of a solution of MgSO 4 in
water. (From Lewis, G.N. and Randall, M., Thermodynamics, McGraw-Hill, New York; Barrow, G.A.,
Physical Chemistry, 5th Edn., McGraw-Hill, New York, 1988. With permission.)
reagent which then affects another reagent. In the spirit of our emphasis on essentials, we limit our
treatment here to binary solutions (again). We should pause to appreciate that solutions are
wondrously homogeneous. Once dissolving occurs, a true solution is essentially immune from
settling of the solute due to gravity. If molecules were just like wooden blocks, we could imagine
that the total volume of a solution would be the simple sum of the volumes of the two components,
the solvent and the solute. In Figure 6.7a [6], we see such a situation where ammonia molecules are
dissolved in water. In this case, the total volume is the simple sum of the water molecules and the
ammonia molecules. Note that in this case both water and ammonia molecules are electrically
neutral, so the volume effect is relatively free of electrical interactions even though there is
a chemical interaction in the form of an equilibrium,
NH 3 þ H 2 O NH 4 OH (NH 4 ) þ (OH) :
þ
!
!
We know from other courses in chemistry that
þ
5
ffi 1:8 10 ,
½ NH 4 OH
½
K i ¼
[NH 4 OH]
so in fact there are only small amounts of charged ions in this weakly basic solution. Although both
water and ammonia molecules are polar, even the dipole moments are similar with m ffi 1:85 D
H 2 O
and m ffi 1:47 D, so the sum-of-blocks works very well. A dipole of 1 Debye unit (1 d)isdefined
NH 3
to be an electrical vector due to a charge separation of (þ1) ( 1) electron units by a distance of 1 Å.
Thus 1 d ¼ 10 18 statcoul cm or 10 10 esu angstroms, a non-SI unit. We will discuss dipole
moments further in a later chapter but you may have encountered dipole moments in organic
chemistry? In Figure 6.7b [6], we see a slightly different and mysterious situation when MgSO 4
is dissolved in water. The solution volume actually contracts for dilute solutions. This is believed to
be due to the attraction of the water dipole moments to the charged species Mg 2þ and [SO 4 ] ,
2
which causes attractive packing of the ions, at least in the primary solvent shell. Eventually the
volume increases as more and more MgSO 4 is added to the fixed amount of water (1000 g H 2 O).
In Figure 6.8 [6], we see an amazing situation where the ethanol molecules dissolved in water
have an aliphatic, hydrophobic alkane portion of the molecule as well as the polar –OH end of the
molecule. Thus, ethanol can simultaneously repel water molecules from the aliphatic part of
the molecule while at the same time participating in the H-bonding interactions with the water
molecules. Thus, we see that in an ‘‘open system’’ where various amounts of solute are added to a