Page 232 - Essentials of physical chemistry
P. 232
194 Essentials of Physical Chemistry
Auger emission (x-ray fluorescence)
(part of electron microscope)
200 keV
(DC)
e ee
Bohr model
z
Fe 2
n
–
E(n, z)=–( ) (13.605 eV)
n
2
z
r(n, z)=(–) (0.529177 Å)
115 VAc
12,398
ΔE (eV)=
λ(Å)
Vaccum
–
e
LiF plate diffraction
(Mo–Krange)
X-ray
Film for x-ray λ
(nλ=2d sin θ
X-ray
for crystal)
Stage
–
– – e
e
e
–
e
–
e
Partition
Film for electron image
–
e
S
–
+Z
e
K-shell
Auger
emission
X-ray
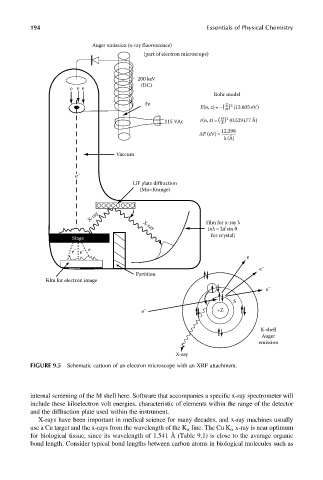
FIGURE 9.5 Schematic cartoon of an electron microscope with an XRF attachment.
internal screening of the M shell here. Software that accompanies a specific x-ray spectrometer will
include these kiloelectron volt energies, characteristic of elements within the range of the detector
and the diffraction plate used within the instrument.
X-rays have been important in medical science for many decades, and x-ray machines usually
use a Cu target and the x-rays from the wavelength of the K a line. The Cu K a x-ray is near optimum
for biological tissue, since its wavelength of 1.541 Å (Table 9.1) is close to the average organic
bond length. Consider typical bond lengths between carbon atoms in biological molecules such as