Page 233 - Essentials of physical chemistry
P. 233
Basic Spectroscopy 195
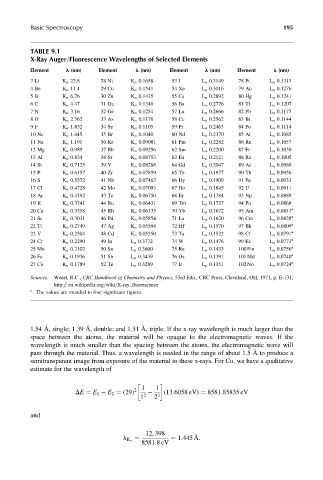
TABLE 9.1
X-Ray Auger=Fluorescence Wavelengths of Selected Elements
Element l (nm) Element l (nm) Element l (nm) Element l (nm)
3Li K a 22.8 28 Ni K a 0.1658 53 I L a 0.3149 78 Pt L a 0.1313
4Be K a 11.4 29 Cu K a 0.1541 54 Xe L a 0.3016 79 Au L a 0.1276
5B K a 6.76 30 Zn K a 0.1435 55 Cs L a 0.2892 80 Hg L a 0.1241
6C K a 4.47 31 Ga K a 0.1340 56 Ba L a 0.2776 81 Tl L a 0.1207
7N K a 3.16 32 Ge K a 0.1254 57 La L a 0.2666 82 Pb L a 0.1175
8O K a 2.362 33 As K a 0.1176 58 Ce L a 0.2562 83 Bi L a 0.1144
9F K a 1.832 34 Se K a 0.1105 59 Pr L a 0.2463 84 Po L a 0.1114
10 Ne K a 1.445 35 Br K a 0.1040 60 Nd L a 0.2370 85 At L a 0.1085
11 Na K a 1.191 36 Kr K a 0.09081 61 Pm L a 0.2282 86 Rn L a 0.1057
12 Mg K a 0.989 37 Rb K a 0.09256 62 Sm L a 0.2200 87 Fr L a 0.1030
13 Al K a 0.834 38 Sr K a 0.08753 63 Eu L a 0.2121 88 Ra L a 0.1005
14 Si K a 0.7125 39 Y K a 0.08288 64 Gd L a 0.2047 89 Ac L a 0.0980
15 P K a 0.6157 40 Zr K a 0.07859 65 Tb L a 0.1977 90 Th L a 0.0956
16 S K a 0.5372 41 Nb K a 0.07462 66 Dy L a 0.1909 91 Pa L a 0.0933
17 Cl K a 0.4728 42 Mo K a 0.07093 67 Ho L a 0.1845 92 U L a 0.0911
18 Ar K a 0.4192 43 Tc K a 0.06750 68 Er L a 0.1784 93 Np L a 0.0889
19 K K a 0.3741 44 Ru K a 0.06431 69 Tm L a 0.1727 94 Pu L a 0.0868
20 Ca K a 0.3358 45 Rh K a 0.06133 70 Yb L a 0.1672 95 Am L a 0.0847 a
21 Sc K a 0.3031 46 Pd K a 0.05854 71 Lu L a 0.1620 96 Cm L a 0.0828 a
22 Ti K a 0.2749 47 Ag K a 0.05594 72 Hf L a 0.1570 97 Bk L a 0.0809 a
23 V K a 0.2504 48 Cd K a 0.05350 73 Ta L a 0.1522 98 Cf L a 0.0791 a
24 Cr K a 0.2290 49 In L a 0.3772 74 W L a 0.1476 99 Es L a 0.0773 a
25 Mn K a 0.2102 50 Sn L a 0.3600 75 Re L a 0.1433 100 Fm L a 0.0756 a
26 Fe K a 0.1936 51 Sb L a 0.3439 76 Os L a 0.1391 101 Md L a 0.0740 a
27 Co K a 0.1789 52 Te L a 0.3289 77 Ir L a 0.1351 102 No L a 0.0724 a
Sources: Weast, R.C., CRC Handbook of Chemistry and Physics, 53rd Edn., CRC Press, Cleveland, OH, 1971, p. E-131;
http:== en.wikipedia.org=wiki=X-ray_fluorescence
a
The values are rounded to four significant figures.
1.54 Å, single; 1.39 Å, double; and 1.31 Å, triple. If the x-ray wavelength is much larger than the
space between the atoms, the material will be opaque to the electromagnetic waves. If the
wavelength is much smaller than the spacing between the atoms, the electromagnetic wave will
pass through the material. Thus, a wavelength is needed in the range of about 1.5 Å to produce a
semitransparent image from exposure of the material to these x-rays. For Cu, we have a qualitative
estimate for the wavelength of
1 1
2
DE ¼ E 1 E 2 ¼ (29) (13:6058 eV) ¼ 8581:85835 eV
1 2 2 2
and
12, 398
¼ 1:445 A ˚ :
l K a ¼
8581:8 eV